12 April 2020
The journey to the first phase I clinical trial in Saudi Arabia
© Youssef Khalil
After a two-day discussion on how to develop clinical trials in the kingdom to develop the national pharma, biomedical and biotechnological fields, the forum concluded with a session on Saudi Arabia’s first phase I clinical trial, using the model as a success story to draw on.
“The launch of the first phase I clinical trial in Saudi Arabia was a dream, and now we see it coming true,” said Majed Al Ghoribi, chairman of the infectious diseases unit at KAIMRC.
KAIMRC’s head of strategy and business development, Abdelali Al Haoudi, explained that launching this trial is a cornerstone for the kingdom’s clinical development. “Having a phase I clinical trial unit that is ready to conduct other phase I clinical trials is a very important platform to push other trials forward, not only for KAIMRC clinicians and investigators, but for other national entities as well,” he said.
Leading the battle against MERS-CoV
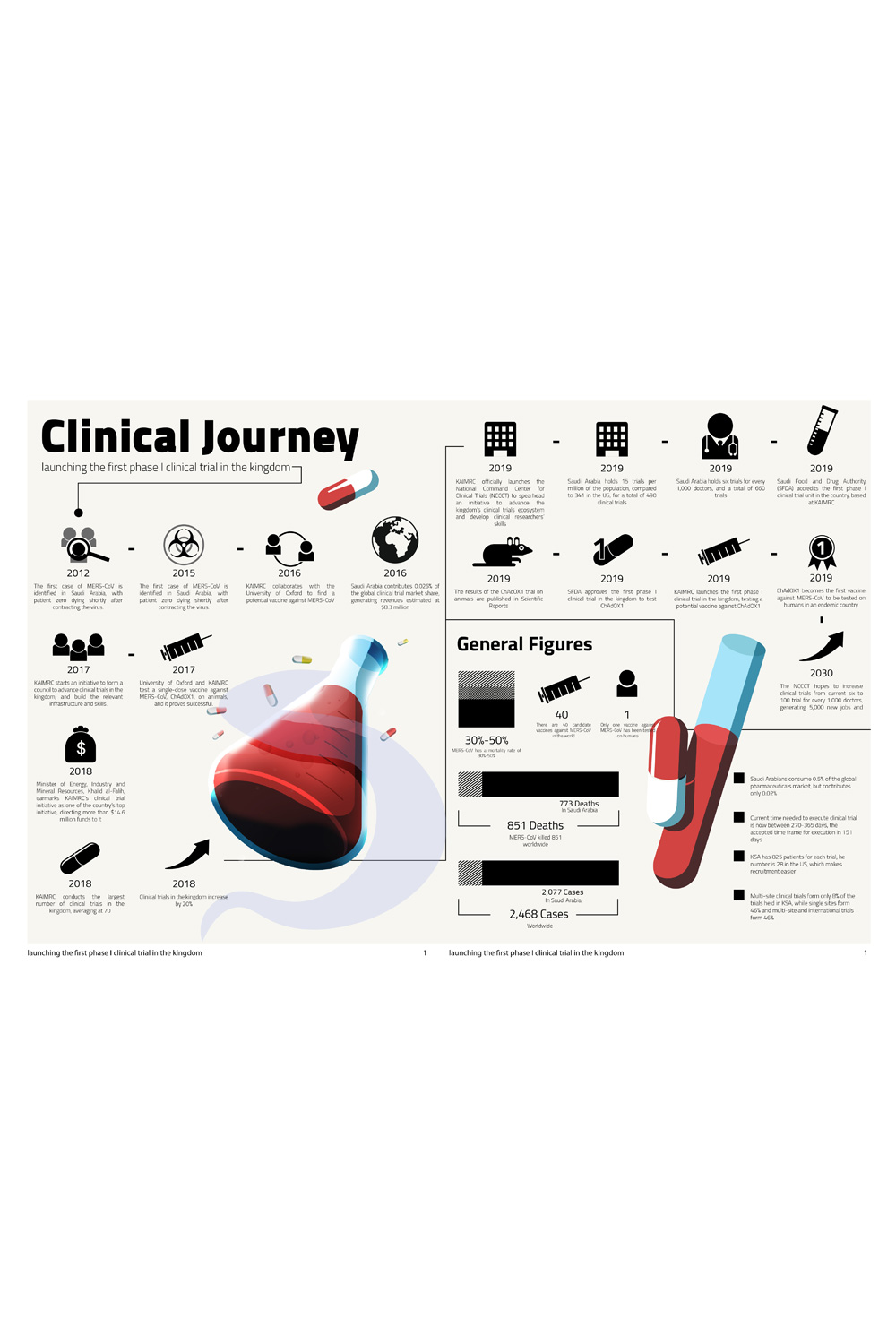
In 2016 KAIMRC and the University of Oxford collaborated to develop a single-dose potential vaccine against the Middle East respiratory syndrome coronavirus (MERS-CoV). The vaccine, ChAdOX1, has already been tested on camels and has proven safe and efficient. After Saudi Food and Drug Authority (SFDA) approval, the vaccine moved into in-human clinical trial phase in December 2019, kicking off in the UK first, then in Saudi Arabia. It is the first MERS-CoV vaccine to be developed in an endemic country, and if successful, would be the only vaccine that requires a single dose.
MERS-CoV affects more than 200 people in Saudi Arabia annually, and has a mortality rate of 30% to 50%. Globally, MERS-CoV has affected 2,468 people worldwide, killing 851. Around 80% of reported cases are in Saudi Arabia, with numbers reaching 2,077 cases since it was first discovered in the kingdom in 2012, and deaths reaching 773.
ChAdOX1 is one of 40 potential vaccine candidates, but only the second to be tested on humans. Four out of these 40 have been developed in Saudi Arabia. While there are no current proven vaccines against MERS-CoV, the strongest candidate so far also requires two doses to reduce the virus load and provide partial protection. This means that if proven effective in humans, ChAdOX1 could provide new hope for the highly infectious virus that spreads from camels to humans. “There are three leading vaccines against MERS-CoV, ChAdOX1 is one of them,” said Naif Al Harbi, head of the research quality and safety department at KAIMRC and one of the researchers involved in the study.
Al Harbi added that the vaccine was developed to be used on camels and humans to stop the virus in its tracks, but that meant overcoming logistic challenges. “Sampling 40 camels every day was a huge task,” said Al Harbi. The vaccine, which is based on a chimpanzee adenoviral viral vector, was tested in mice, then dromedary camels, and the results of the research were published in Scientific Reports1. The vaccine has also successfully reduced virus replication in camels, and could be used on camels and humans.
“Successful vaccination of camels would reduce virus circulation in this animal host and thereby reduce human exposure, leading to a reduction in the number of human cases,” said the study authors. “In addition, developing a vaccine for humans and vaccinating humans at risk, such as camel keepers and healthcare workers, would further control the number of human infections.”
A three-year journey
The trial is illustrative of the advancements that Saudi Arabia has achieved over the last few years, led by the efforts of the National Command Center for Clinical Trials (NCCCT).
“KAIMRC has worked extremely hard over the last couple of years to achieve this,” said Haoudi. “This launch means the ability to conduct initial trials will be established, and the quality and quantity of the trials will improve.”
Majed al Jeraisy, the chairman of KAIMRC’s research office, calls ChAdOX1 a “success story” of researchers and the SFDA collaborating to get the study into the trial stage. “I am very optimistic that this will open the door to more phase I clinical trials to come to the country,” he said, adding that he is confident that the approval process will be shortened after lessons learned through the initial attempt.
KAIMRC started the approval process with the SFDA in 2018, and started advertising for recruitment a year later. “Without the SFDA and their encouragement, this country wouldn’t have been able to have a phase I clinical trial,” said Al Harbi. “Vaccine development usually takes 15 to 30 years, so this was remarkably fast.”
Participants stressed the importance of collaboration in the success of the ChAdOX1 study, and to any potential trial, explaining that universities, government entities, strategic sectors and research centres need to form partnerships.
“The time has passed for one single investigator to launch a study or an invention,” Haoudi said. “Now, it is about the collective efforts of a number of individuals and institutions, and this is the case here.”
References
- | article