10 February 2019
It is particularly hard to develop vaccines for viruses that have RNA as their genetic material, such as those responsible for flu, Ebola and hepatitis C. Their average mutation rates are estimated to be about 100 times greater than those for DNA viruses, mainly due to the lack of genome repair mechanisms. This allows them to evolve rapidly, evade the immune system and become drug-resistant. It also explains why vaccines that are effective during one flu season might be ineffective the next.
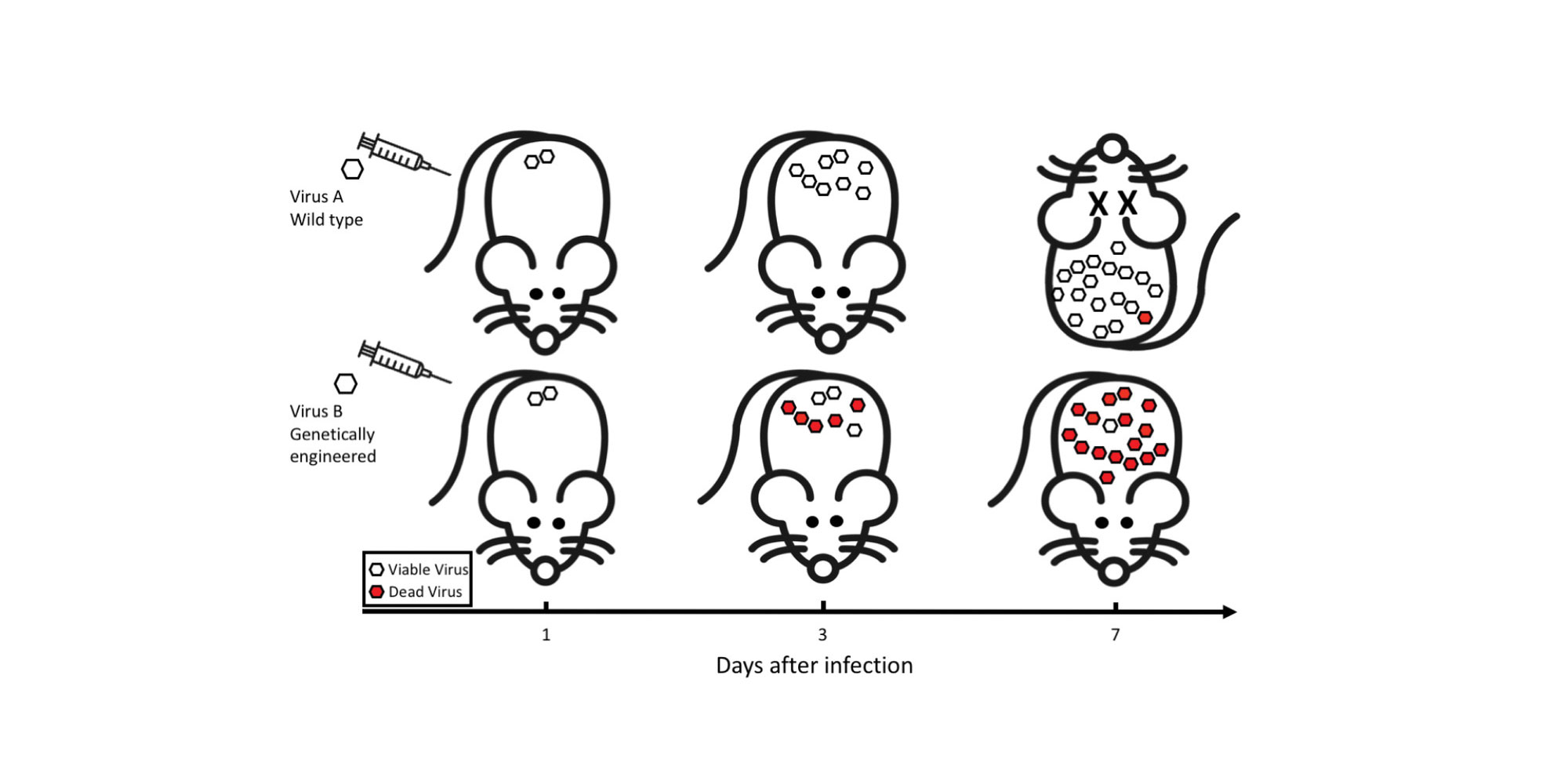
Marco Vignuzzi and his team at the Institut Pasteur in Paris have genetically engineered two very different viruses that are both one mutation away from generating a potentially lethal signal in the part of their genetic sequence that halts protein synthesis, thus preventing replication in the host cell.
“We have devised a way to beat them at their own game,” explains Gonzalo Moratorio, lead author of the study published in Nature Microbiology.
The authors designed a fully functional Coxsackie B3 enterovirus and an influenza A virus, in which a single mutation in one of more than 100 key regions of their genetic sequence would severely compromise their survival.
The attenuation of the viruses was first assessed in cells. Although the ability of the engineered strains to replicate was not significantly different from wild-type control strains, they were more sensitive to deleterious mutations when they were exposed to mutagenic conditions, and virus titres fell up to 100 fold.
When the viruses were tested in mice, not only were they less virulent, but they triggered the production of antibodies that protected the mice from infection with wild-type strains.
The degree of virus attenuation was further increased when the authors engineered an error-prone version of the viral protein responsible for replicating the virus’ genetic code. Introducing this low-fidelity RNA polymerase accelerated the generation of detrimental mutations and further reduced virus infectivity. Mice infected with this ‘optimal suicidal virus’ developed neutralizing antibodies that were able to protect them against lethal challenges with the wild-type viruses.
“We were very surprised by the finding that a virus that is more prone to mutate, can actually increase survival,” said Moratorio.
Further work is under way to better understand the immune response that is triggered by these virus strains and determine how broadly this ground-breaking approach to antiviral vaccine development can be applied.
References
- Moratorio, G., Henningsson, R., Barbezange, C., Carrau, L., Borderia, A.V. et al. Attenuation of RNA viruses by redirecting their evolution in sequence space. Nature Microbiology 2, 17088 (2017). | article