13 December 2016
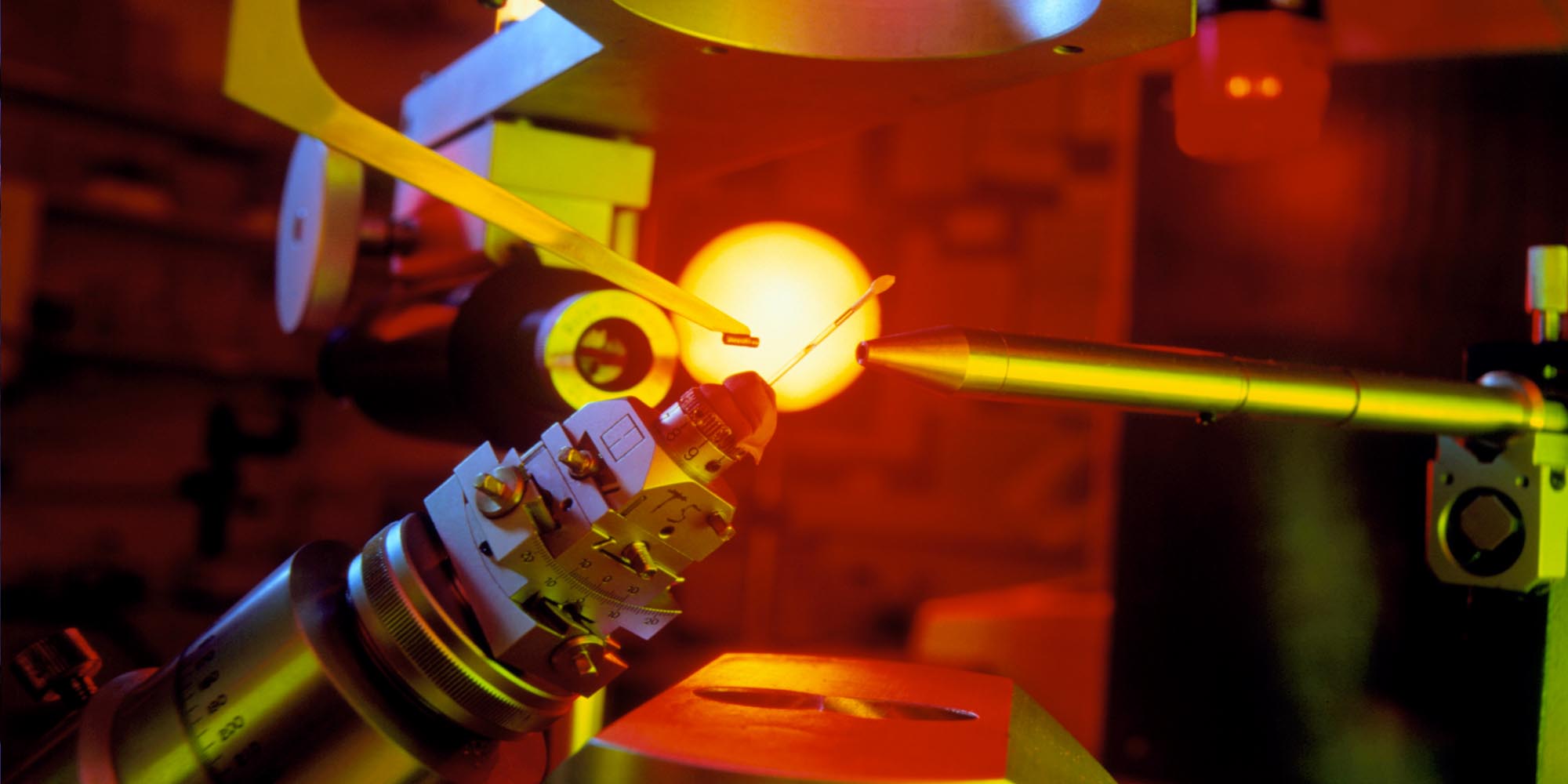
Advances in nanotechnology are often inspired by designs found in nature. Tiny molecules that can change their shape in response to stimuli and swim through the body, mimicking bacteria, might one day help scientists develop novel, targeted drug delivery systems. Based on this principle, KAIMRC researchers have developed nanowires that bend in response to visible wavelengths of light and revert back in the presence of UV light.
“We were inspired by the way bacteria propel themselves through liquid using their flagella, or tails, utilizing chemical energy as fuel,” explains Rabih Al-Kaysi, the KAIMRC materials chemist who led the project in collaboration with scientists at the University of California Riverside in the US. “We aimed to mimic nature by fabricating flagella-like nanowires that could move using light as fuel instead.”
Scientists have already developed molecular crystals that respond to light of different wavelengths and undergo conformational changes. These ‘photomechanical’ properties are highly desirable, particularly if the shape-changes are reversible, so that a motion can be repeated or the molecule can be re-used. There are two types of reversibility: T-type (in which a molecule induced to change by light slowly reverts to its original shape after a time) or P-type (where shape-change is controlled in both directions by exposure to light of different wavelengths).
“Until now, researchers were unable to create a nanoscale light-activated P-type actuator – a component that converts energy into mechanical motion,” says Al-Kaysi. “By synthesizing the appropriate organic molecule, we have generated the first P-type photomechanical actuators with nanometer thickness.”
The researchers synthesized their novel molecule, called 9DVAM, by reacting a derivative of the hydrocarbon anthracene with an organic compound called malononitrile and fabricating molecular crystal nanowires that bent and straightened repeatedly under light stimulation. Further analysis of 9DVAM’s structural changes using X-ray diffraction revealed that the molecule was switching between two isomer shapes (different orientations of the atoms within the molecule) in response to light.
“These conformational changes offer the largest possible displacement in the molecule, meaning maximum energy is generated,” explains Al-Kaysi. “This lays the foundations for building molecular switches, or making artificial robotic flagella that could be attached to drug-loaded carriers, allowing them to be steered towards target tumour sites.”
“We’re at the early stages of developing systems that can be used inside the body, where it is dark. By utilising clever synthesis, we could develop molecules activated using near-infrared radiation, which can penetrate the body.”
References
Zhu, L., Tong, F., Zaghloul, N., Baz, O., Bardeen, C.J. et al. Characterization of a P-type photomechanical molecular crystal based on the E – Z photoisomerization of 9-divinylanthracene malonitrile. Journal of Materials Chemistry C (2016). | article