8 December 2019
Recent findings from a cohort study of patients with a rare hereditary immunodeficiency disorder reveal insights into a gene’s role in immune function, as well as possible treatment avenues. The study is the first to characterize the effects of complete RIPK1 (a signalling protein) deficiency in humans.
Hundreds of genes have already been linked to primary immunodeficiency (PID) disorders, and an international team of researchers led by Sergey Nejentsev, at the University of Cambridge, embarked on a dragnet to identify additional causative mutations. They sequenced the full complement of genes from 48 patients who had experienced recurring, severe infections throughout their lives as a consequence of PID.
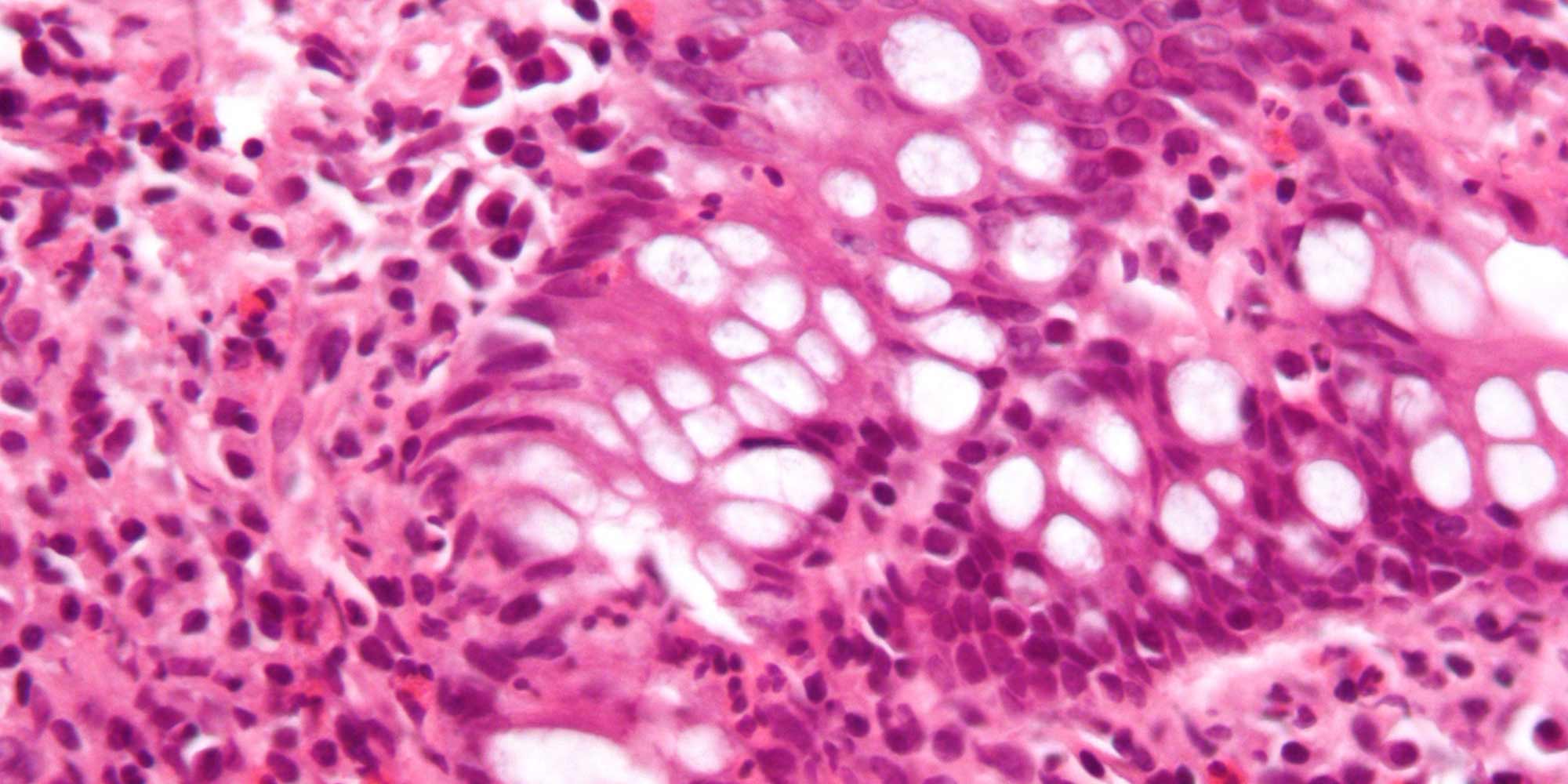
Four of these 48 people had mutations that essentially disabled the same gene, encoding a RIPK1. In addition to being infection-prone, all four also sufferedfrom arthritis and inflammatory bowel disease (IBD) — conditions associated with immune dysfunction. Previous studies have indicated that RIPK1 acts as a master regulator that controls the inflammatory response and cellular survival, but this is the first report to date of humans with RIPK1 deficiency.
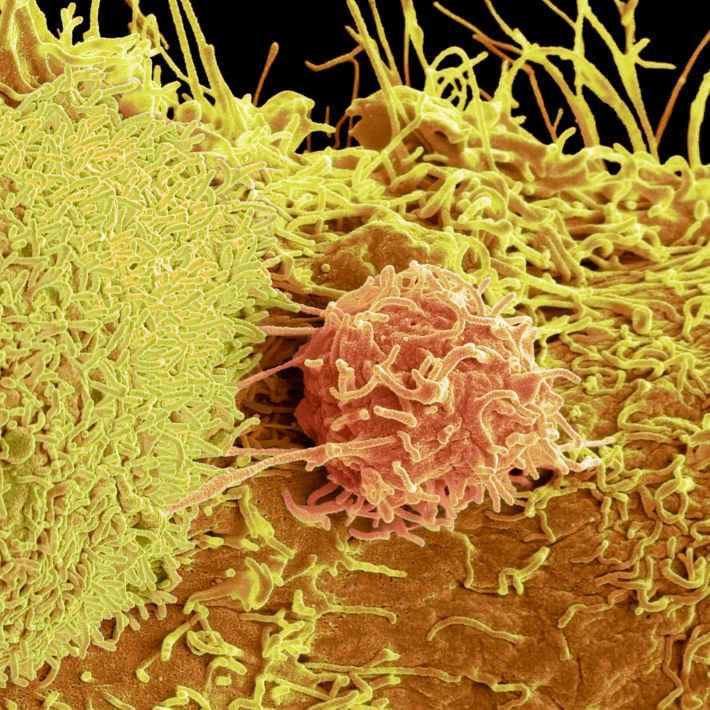
Working with both patient-derived blood cells and genetically modified human cell lines, Nejentsev’s team found that RIPK-deficiency debilitates the cellular response to molecules that normally trigger a strong immune response. These findings, which were published in Science, could explain why the patients failed to fend off infection. The researchers also identified deficiencies in the production of certain signalling proteins that have previously been linked to the onset of IBD.
These results are in striking contrast to previous data from RIPK1-deficient mice. The mice experience extensive inflammation and cell death early in development, and perish soon after birth. In contrast, two of the affected individuals had reached adolescence by the time of this study, illustrating thelimitations of mouse models for predicting the clinical impact of genetic mutations.
Importantly, the study also identified potential avenues for the treatment of this form of PID. For example, one of the four patients achieved considerable — but not complete — recovery after receiving a stem cell transplant that enabled production of healthy, RIPK1-expressing blood cells. The researchers also determined that excessive production of an immune signalling protein called IL-1β is a common feature of RIPK1 deficiency. Based on this finding, they propose that drugs that inhibit this protein could potentially alleviate the immune symptoms in these patients.
References
Cuchet-Lourenço, D., Eletto, D., Wu, C., Plagnol, V., Papapietro, O. et al. Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation Science 361, 810–813 (2018). | article