7 July 2020
Mantle-cell lymphoma is a rare and highly aggressive form of B-cell non-Hodgkin’s lymphoma. Patients often relapse after treatment, or their cancer becomes resistant to BTK inhibitors, a leading therapy for the disease.
For the first time, an international team of scientists, led by Michael Wang at the University of Texas in Houston, US, have used an immunotherapy approach to treat patients with advanced mantle-cell lymphoma.
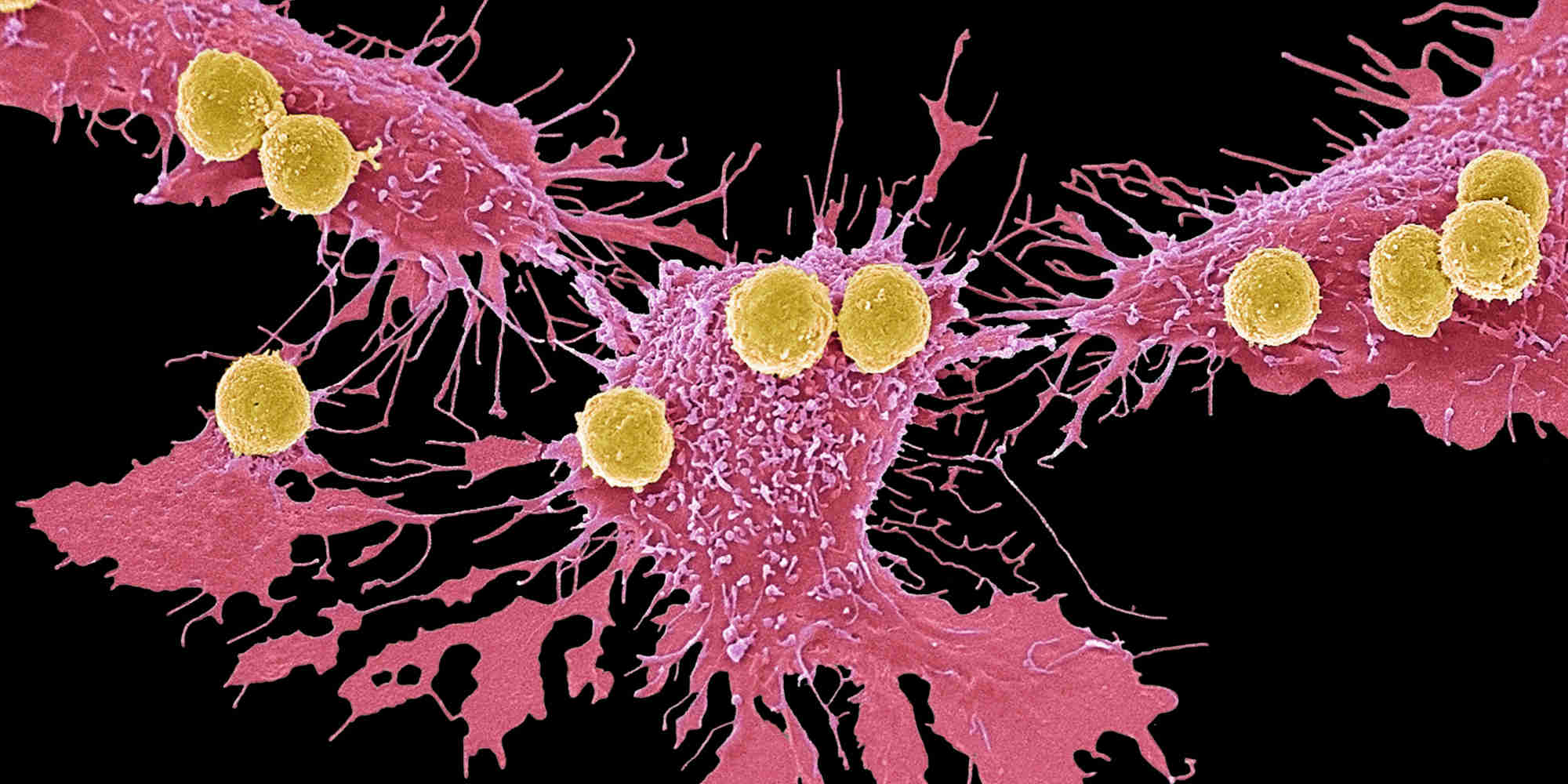
The team’s phase II clinical trial was conducted at 20 sites across the US and Europe between 2016 and 2019. The researchers used a form of CAR T-cell therapy, where samples of immune cells called T cells are collected from patients. The cells are then altered in the lab to enhance the expression of a specific protein that helps the T cells bind to particular cancer cells. Large numbers of these ‘super-charged’ immune cells are grown in the lab, then injected back into the same patient. This induces a more potent attack by the patient’s own immune system against cancer.
In total, 74 patients from the United States and Europe were enrolled in the study, and 68 patients completed the treatment. Most of the patients had received at least three previous types of treatment for their lymphoma, and had either relapsed or their cancer had become treatment resistant. The team arranged for an independent radiology review committee to assess the clinical response of each patient to the therapy.
In promising results, 93% of trial patients responded to the therapy, and 67% experienced complete remission. Those patients that responded well showed robust T-cell levels and reduced cancer cell activity at their first assessment after treatment. Follow-ups with patients a year after the treatment had run its course showed that 57% remained in remission.
However, CAR T-cell therapy is known for its potentially severe side-effects and toxicity, and almost all the trial cohort experienced adverse effects, including low red blood cell counts and infections. No patients died as a result of the side-effects, although some were seriously ill for several weeks following the administration of the therapy.
“Patient-reported outcomes suggested no long-term quality-of-life defects after [therapy] receipt,” state the researchers in their paper published in The New England Journal of Medicine in April 2020. “This trial showed that a single infusion of KTE-X19 CAR T-cell therapy was capable of inducing durable remissions in patients with relapsed or refractory mantle-cell lymphoma.”
References
- Wang, M., Munoz, J., Goy, A., Locke, F.L., Jacobson, C.A., et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. The New England Journal of Medicine 382 (2nd April 2020) | article