4 November 2020
Men of African ancestry are more than twice as likely as other populations to develop prostate cancer. Genetic components partly contribute to this disparity, but their role is ill-defined. An international team has now determined how a rare genetic variant prevalent in men of African ancestry increases their risk.
Several variants at chromosome region 8q24 have been linked with different types of cancer. The region is home to several genes that are more strongly expressed in prostate tumors, including multiple long non-coding genes (lncRNAs) and the protein-coding proto-oncogene MYC, a gene that can contribute to cancer when mutated.
The non-coding region of 8q24 also contains rs72725854, a rare genetic variant with a pivotal role in the elevated prostate cancer risks in men of African ancestry. However, the relationship between rs72725854 and its neighboring genes has been unclear.
The team devised an approach relating three-dimensional chromatin structure to transcriptional regulation in order to establish the functional relevance of rs72725854. This enabled them to reveal a mechanism behind the heightened prostate cancer risk. “This is the first functional study that mechanistically links a rare variant in non-coding genome with disease susceptibility,” says team leader, Dimple Notani, from the Tata Institute for Fundamental Research in India.
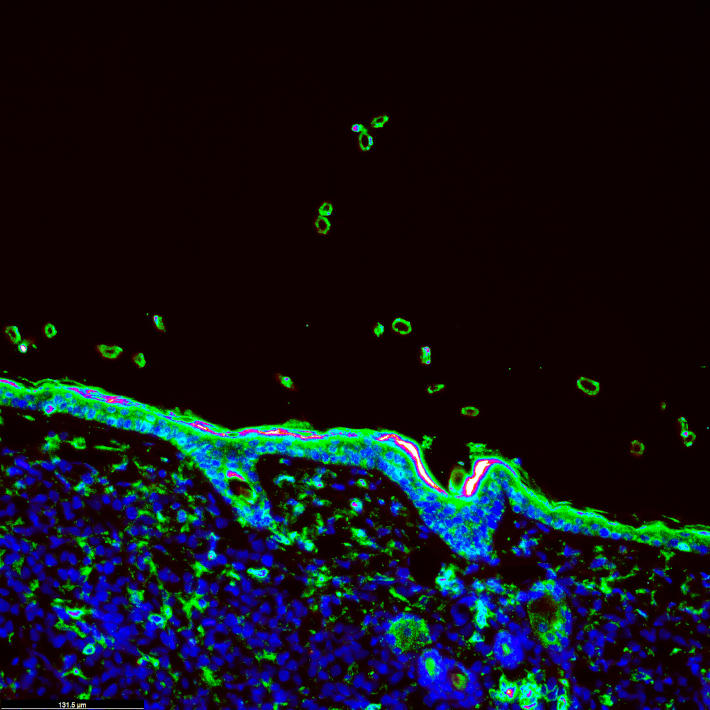
DNA-protein interaction analysis revealed that the region containing rs72725854 acts as a prostate cancer-specific enhancer. The region was inactive in healthy cells and turned into an extremely active androgen-responsive enhancer in prostate cancer cells.
The researchers found that, unlike its non-risk counterparts, the risk allele of rs72725854 amplified the activity of the enhancer by binding to the transcription factor SPDEF, a sequence-specific DNA-binding protein that controls transcription rate. This promoted the response of the enhancer to androgen stimulation. Structural analysis showed that the enhancer physically interacted with lncRNAs and MYC promoters by forming chromatin loops, which increased the expression of the genes. The proximity of allele-bound SPDEF augmented the activity of the enhancer, boosting the expression of nearby lncRNAs and MYC.
“This study helps us better understand the causes of complex diseases, such as prostate cancer, which is valuable for designing diagnostic tools and therapeutics,” Notani says.
The team is currently planning to apply their approach to understand risk factors to other complex diseases, such as the susceptibilities of the Indian population to metabolic diseases like diabetes. “That would pave the way for more efficient diagnostic tools and, ultimately, treatments for these diseases,” Notani says.
References
- Walavalkar, K., Saravanan, B., Singh, A.K, Jayani, R.S., Farooq, U. et al. A rare variant of African ancestry activates 8q24 lncRNA hub by modulating cancer associated enhancer. Nature Communications 11, 3598 (2020). | article