14 August 2016
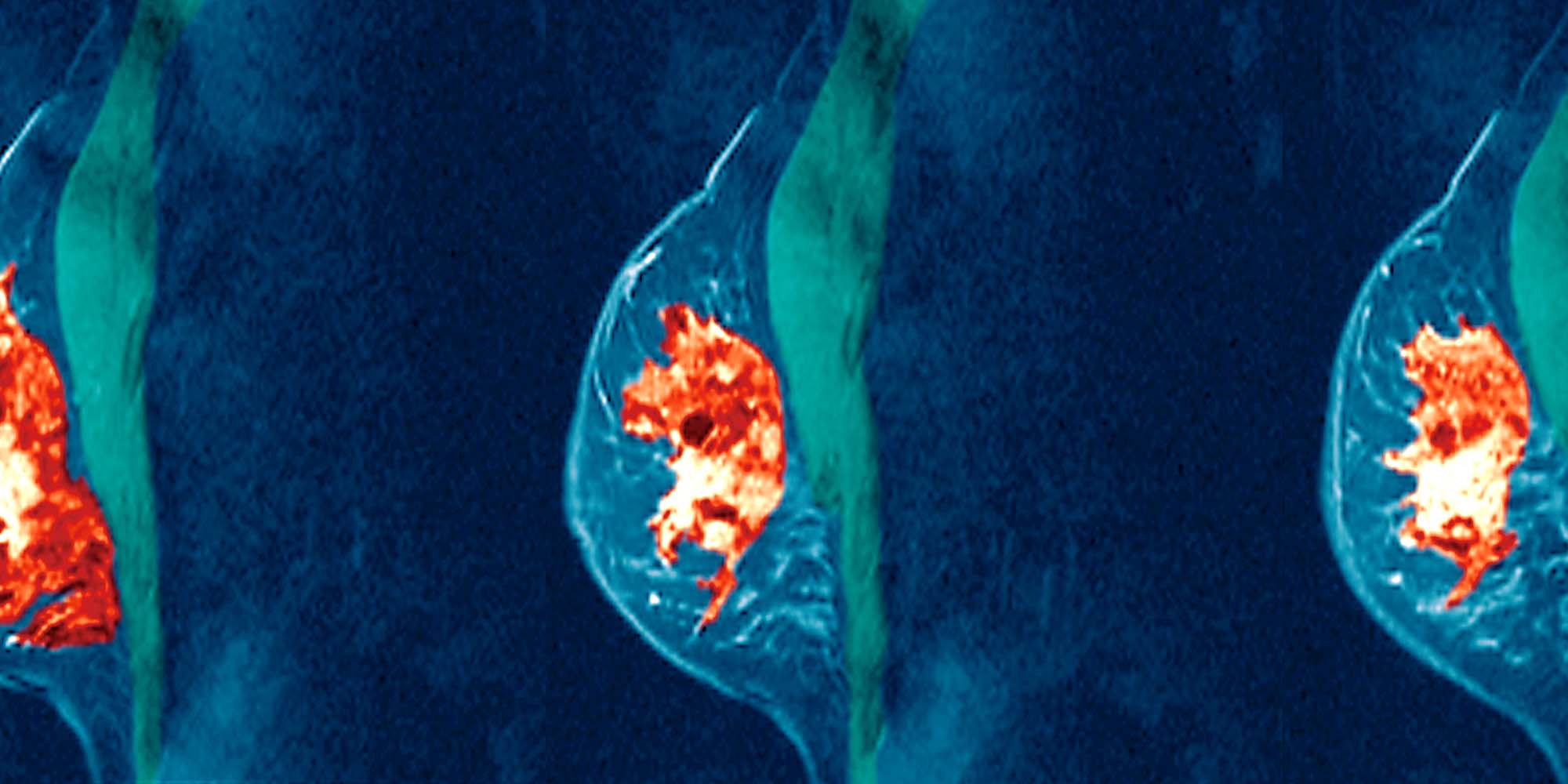
Breast cancer is the most common type in women worldwide. When confined within the breast and the lymph nodes of the armpit it is referred to as “locally advanced breast cancer” (LABC). These patients receive a combination of anti-cancer drugs before surgery to reduce the volume of the tumour and to improve chances of survival. When successful, breast conservation, rather than mastectomy, is possible.
Researchers in Saudi Arabia, Kuwait, Egypt and the United Arab Emirates conducted a clinical trial of a new pre-surgery chemotherapy treatment on 80 LABC patients to test its effectiveness and safety. The trial showed promising results, even in aggressive types of LABC.
In chemotherapy, a cocktail of drugs is used to attack cancer cells. However, it is hard to know which combination leads to the best results, with the lowest toxicity. In this trial, LABC patients received a 24-week treatment of the chemotherapeutic agents FEC100, cisplatin and docetaxel. Patients whose cancer cells had a high number of human epidermal growth factor 2 (HER2) receptor proteins were also given trastuzumab, which targets these receptors. HER2-positive breast cancers tend to grow faster and are more likely to spread and recur compared to HER2-negative ones.
A quarter of the patients could not complete the course because of unacceptable toxicity. But, the invasive tumour cells were completely eliminated from the breast alone of 4% of the remaining women, the armpit alone in 32%, and from both breast and armpit in another 32%. “It is an encouraging result, considering that almost half the patients in our hospital have LABC and begin chemotherapy when the tumour is relatively large,” says oncologist Omalkhair Abulkhair from Saudi Arabia’s Ministry of National Guard –Health Affairs.
Around 15% of breast cancers are classified as “triple negative” because they do not have oestrogen or progesterone receptors on their cell membranes and do not overexpress HER2. Triple negative tumours are difficult to treat since most chemotherapies target these receptors specifically. Moreover, triple negative cancers tend to occur more often in younger women and grow more quickly than other types of breast cancer.
The trial achieved good results with these more aggressive types of cancers: 36% of the triple-negative breast cancers were eradicated, as well as 62% of the HER2-positive, hormone receptor–negative group. However, “further studies are needed to improve the response of HER2-negative, hormone receptor-positive patients and to evaluate the efficacy of a shorter therapy,” says Omalkhair Abulkhair.
References
AL-Tweigeri, T., AlSayed, A., Alawadi, S., Ibrahim, M., Ashour, W. A multicenter prospective phase II trial of neoadjuvant epirubicin, cyclophosphamide, and 5-fluorouracil (FEC100) followed by cisplatin-docetaxel with or without trastuzumab in locally advanced breast cancer. Cancer Chemotherapy and Pharmacology (2016).| article