8 June 2020
Ahmed Alaskar, KAIMRC’s executive director, discusses the centre’s efforts in repurposing research labs and infrastructure to focus on SARS-CoV-2, the virus that causes COVID-19. Like many institutions around the world, KAIMRC is searching for ways to repurpose existing drugs, after isolating and sequencing the COVID-19 virus from a patient in Saudi Arabia. KAIMRC researchers are searching thousands of existing compounds and medications with the help of various cutting-edge technologies. In addition, using computational methods (modeling) we have generated new lead molecules. These new chemical entities (NCE) will be synthesized, and assayed in live virus assay.
Coronavirus is the subject of intense research in Saudi Arabia, and particularly at KAIMRC. You have announced progress in terms of isolating and sequencing the virus. What are some of the research trajectories KAIMRC is exploring?
There are several ways of repurposing medications for COVID-19 and researchers use different technologies to search among thousands of existing compounds and medications. KAIMRC has a few of these technologies.
Can you give an example of these, and how they are helping researchers?
We use molecular modeling, medicinal chemistry and high-throughput drug screening of FDA approved drugs. This will give us an advantage to get the drug to market faster since these drugs already passed through Phase I (Safety and Toxicity) clinical trials.
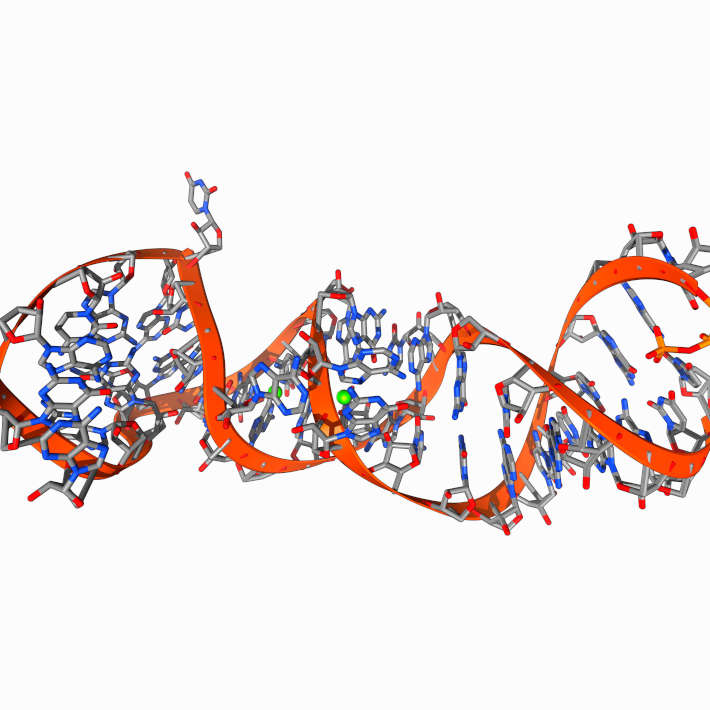
Leading up to one of our clinical trials, for example, we’re using computational biology and bioinformatics to search among existing drugs molecules and see where they bind in the COVID-19 virus or genome, to predict whether they can be used against the disease. Also, we have expressed one of the key enzymes of COVID-19 and testing FDA approved drugs. We are hopeful that we will repurpose a drug molecule through these efforts.
We look at the virus structure in three dimensions and try to see where the medication binds to the virus, and if it binds into a sensitive target of the virus, like envelop a protein, or an enzyme that might kill it. You need to target certain target proteins such as enzymes, genetic codes like RNA or lipids that envelop the virus, of which there are usually multiple, for the computer to try to match up with the drugs in question. That is being done now, and we are coming up with a number of compounds that will be validated in the laboratory, and in planned clinical trials.
Do you have enough Saudi patients for these types of trials?
We do. We’re collaborating with many hospitals in Saudi Arabia to reach as many patients and samples as possible. There are several major projects that are ongoing across the country and some of them are led by institutions other than KAIMRC.
You mentioned that you are trying to repurpose several medications for the treatment of COVID-19 that have been pre-approved by the Saudi Food and Drug Administration (SFDA). Is there a specific medication or a combination of medications that is exceptionally promising?
We are participating in the international clinical trial led by the World Health Organization Solidarity, which tests Remdesivir, Chloroquine, Lopinavir with Ritonavir and Interferon β1b. This trial is enrolling patients in many countries, including in seven hospitals in Saudi Arabia. There is another national trial led by KAIMRC that uses Favipiravir, a medication that was developed by Japanese company Fujifilm Toyama Chemical and approved for certain type influenza in Japan. We have done a computational biology screening and we found Favipiravir a suitable candidate. To follow up on Favipiravir, we have synthesized some analogs of Faviparavir which will be assayed in RNA dependent RNA polymerase (RdRP), a critical enzyme for replication of virus. We are trying Favipiravir in combination with Hydroxychloroquine, a medication that is typically used to prevent and treat malaria.
There is some skepticism about the world’s ability to fast-track a vaccine. We have had other types of coronaviruses such as SARS-CoV and MERS-CoV for years, and there are still no vaccines for either. Why would SARS-CoV2 be any different?
The magnitude of the impact of COVID-19 on the world is much bigger than the others. The impact of SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome) was limited, and hence, the whole world was not as pressed to speed up the development of a vaccine. For MERS, there are three vaccines that have reached clinical trials worldwide. One of them is ours, in collaboration with Oxford University. This potential MERS vaccine is now in phase one clinical trial [and the research project kicked off in 2016]. Considering the amount of time it takes to develop a vaccine, this is actually going faster than usual. However, compared to the efforts to find a vaccine for COVID-19, even this fast-tracked MERS trial is relatively slow. When it comes to COVID-19, the world is at the fastest that we've ever seen in terms of the amount of science that is being developed and shared. The whole world and its resources are just focused on this issue. That inspires hope.
With KAIMRC’s focus on the current pandemic, do you believe that COVID-19 research may be exhausting your funds or taking attention or resources from other important areas of research inside KAIMRC, such as cancer research, for instance? How do you think KAIMRC can strike a balance?
There will definitely be an impact on research areas other than COVID-19, financially and logistically, in terms of resources, and the ability to conduct research, even on the patients themselves. This is definitely affecting everything, and other research will be affected for a while.
References
- | article