15 November 2021
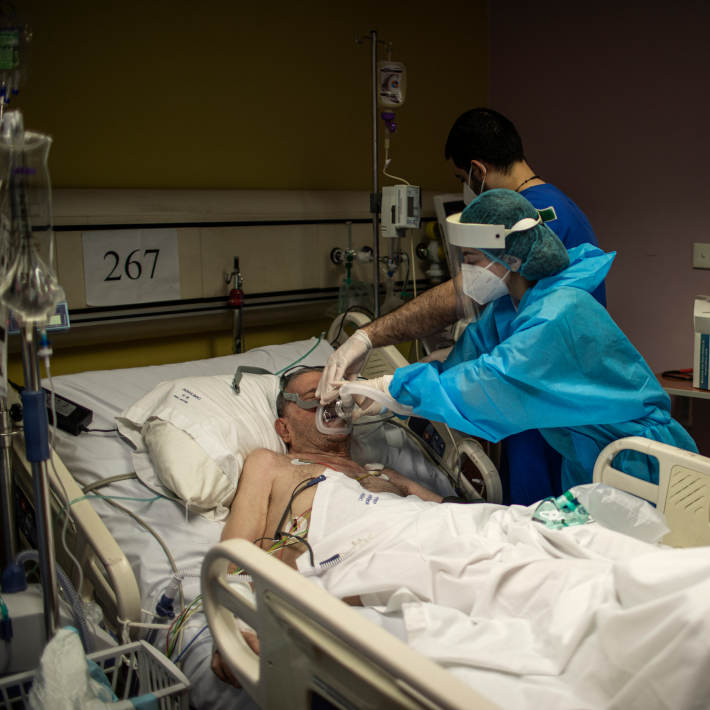
Making use of existing, certified drugs cuts the time it takes to begin therapy in patients, and many clinical trials are examining the use of antiviral drugs to tackle COVID-19.
One such trial, underway in Saudi Arabia under the guidance of Mohammad Bosaeed, an associate director of Research Trial Services in KAIMRC, is examining favipiravir as a potential COVID-19 therapy. The antiviral medication has been shown to improve Ebola survival rates in non-human studies, as well as reducing viral load in influenza patients and speeding up their recovery.
“Early, effective antiviral therapy can help prevent COVID-19 progression to severe illness, especially for patients at high risk, with the additional benefit of decreasing the burden on the healthcare system,” says Bosaeed. “Taking antivirals can also reduce the number of viral particles shed by an individual, which limits the spread of the disease.”
Favipiravir is a potent inhibitor of viral RNA polymerase – the enzyme that kickstarts the replication of viral RNA in host cells. By blocking polymerase activity, the antiviral drug is a valuable tool against RNA viruses such as Ebola and coronaviruses, preventing their spread through the body.
Bosaeed and his team have begun their multi-centre, randomised double blinded clinical trial of favipiravir as a potential treatment for adults with mild COVID-19 symptoms. Around 230 patients have been enrolled so far from different hospitals and clinics in Saudi Arabia.
“We are working closely with all sites involved to mitigate issues with regards to recruitment, and the trial is designed to take patient hesitancy into account,” says Bosaeed. The team aims to recruit more than 500 participants in total unless the Data and Safety Monitoring Board recommends an early termination for safety or efficacy reasons. The team monitors the clinical progression of the patients and tracks the time it takes for each patient to present a negative PCR test.
In previous trials, treatment with favipiravir has proven safe, although the drug can cause some side effects, such as diarrhoea. The researchers will carefully monitor the patients for adverse effects during the trial. Bosaeed acknowledges that, along with the other unknowns in dealing with this novel coronovirus, it is unclear whether the efficacy of treatments (including antivirals) could be affected by future viral mutations.
References
- Bosaeed, M. et al. Multicentre randomised double-blinded placebo-controlled trial of favipiravir in adults with mild COVID-19. BMJ Open 11 (e047495) (2021) | article