19 August 2020
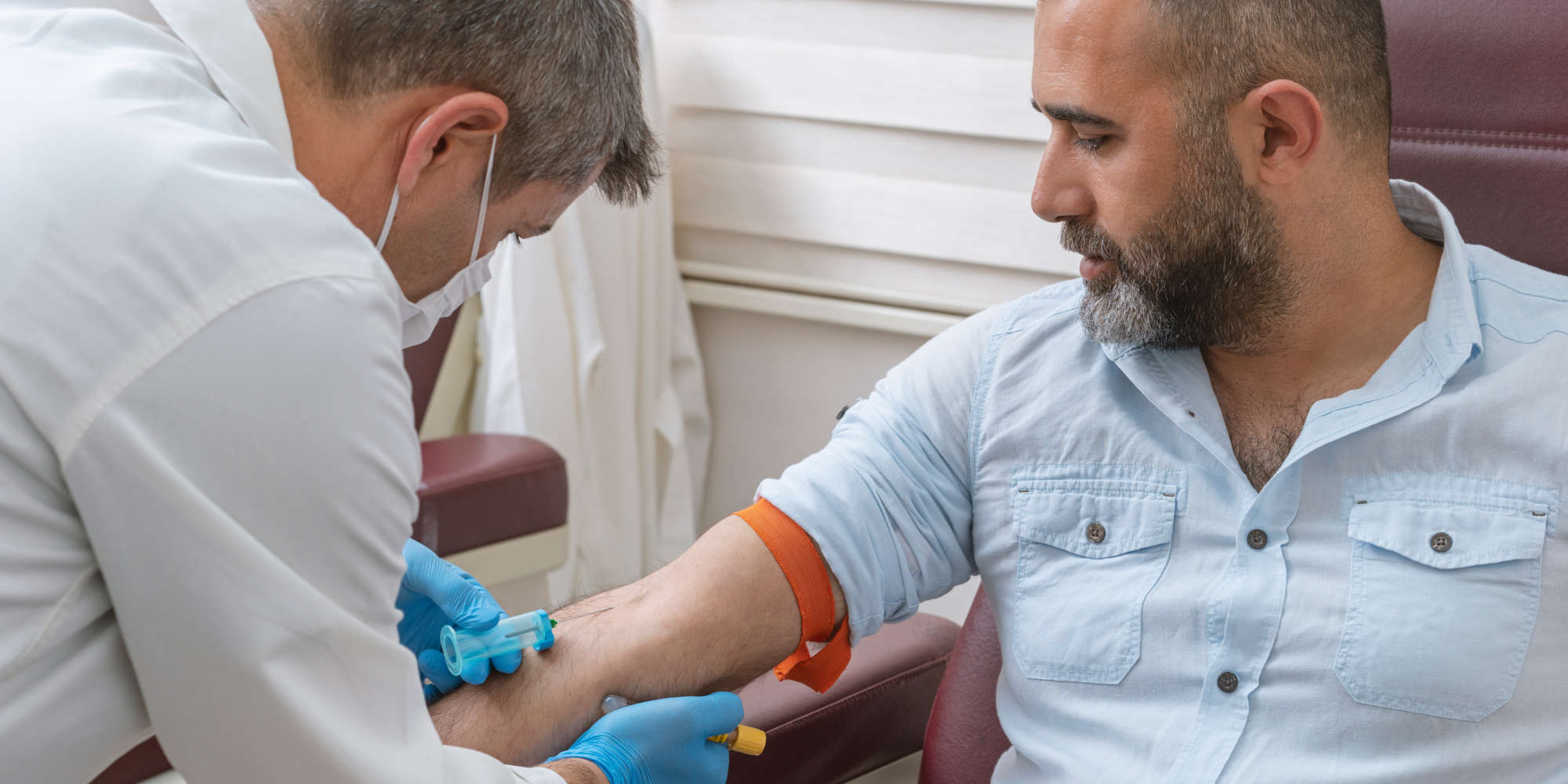
To fully test drugs and vaccines, clinical trials on human volunteers are critical. While many countries around the world have conducted such ‘first-in-human’ (FIH) clinical trials for decades, Saudi Arabia launched their first FIH clinical trial center at KAIMRC, Riyadh, in December 2019. Its first phase I clinical trial is now underway to test a novel MERS-CoV vaccine on healthy volunteers.
FIH clinical trials help scientists confirm the safety of new drugs and vaccines, as well as clarifying their precise mechanisms of action — exactly how a drug works and behaves once it is inside the body. Such FIH trials are preceded by years of work on a specific molecule in the lab and on animals, in order to maximise the chances of a safe, positive and valuable outcome once a drug reaches FIH trial level.
An ongoing challenge for FIH programmes is the need to recruit and retain volunteers. Depending on the drug to be tested, scientists may require healthy subjects, or sometimes trials are conducted on consenting patients who have a specific disease. Access to a willing and diverse selection of people from different backgrounds, age-groups and states of health can be an invaluable asset to clinical researchers. Also, the ability to test new medicines on different nationality groups is of great interest to the wider international research community.
With a new era of FIH trials dawning on Saudi Arabia, Adel Almutairi, Badriah Almutairi, and their team at KAIMRC’s Science and Technology Unit and Clinical Trial Services under Research Office conducted a survey of 657 Saudi nationals to examine their attitudes towards participating in FIH clinical trials. Their findings showed a positive attitude, and a strong willingness to participate, especially among younger respondents.
“In the run up to the establishment of the KAIMRC unit in the kingdom, we began to wonder: Are we ready for this?” says Adel Almutairi. “We wondered if people would pay more attention to FIH clinical trials in general, and if we could tap into that interest to encourage volunteers. We were curious to see if our society would be open-minded and willing to participate in such trials.”
In February 2018, the team distributed an electronic questionnaire that was completed by equal numbers of male and female respondents from a variety of ages and backgrounds. The respondents had indicated an interest in the study on social media, or were approached during the annual cultural and heritage festival in Riyadh (Al Janadriyah). The questions sought to probe the Saudi public’s motivations and willingness to take part in FIH trials, and clarify their stance on participating in such trials.
Almost 72% of participants stated their intention to enroll in trials, and those who had high regard for the country’s healthcare services were more likely to have a positive attitude towards the importance of such programmes. Further, over 80% said that they would take part if their physician suggested it, highlighting a strong respect for the medical profession in the kingdom.
“Previous research studies have shown that a key reason for people across the world participating in clinical trials is altruism – people really want to help others,” says Grant Huang, director of the Cooperative Studies Program at the Department of Veterans Affairs in Washington DC in the United States who was not part of the study. Huang’s expertise lies in designing and conducting national and international clinical trials and large-scale epidemiologic studies. “Many active participants share a desire to benefit mankind, and others have a strong personal interest in medicine and science,” he adds.
The Saudi research shows that “young people (under the age of 30) and single people appeared to be most interested in enrolling in the trials,” says Adel Almutairi. “There might be a certain degree of cultural influence bias among the younger participants since wider perspectives on FIH clinical trials appear to have been shared and discussed rigorously through social media here.”
While the general outlook on FIH clinical trials in Saudi Arabia appears positive, participants in Almutairi’s study also highlighted a number of concerns. The respondents were asked to rank a series of statements according to how strongly they agreed or disagreed with them. Among the ‘concern’ statements, ranked most highly were a fear of the unknown, and a concern that people would be treated like guinea pigs. Respondents (57%) favoured shorter trials of one to three days in length, rather than a week or more. Other factors for concern included conflict with religious, social and moral beliefs.
“It is important to understand that behind it all we are dealing with people - their own beliefs, experiences and other personal factors must be considered,” says Huang. “To shift such beliefs and encourage participation in trials, the general public needs help in understanding the value and role of clinical trials. Governments, scientific and clinical communities, and patients can all play a role in explaining the importance of trial completion. It is also vital to understand the perceptions built around the wider history of clinical trials.”
Based on their study’s findings, the KAIMRC team has put a comprehensive, strategic plan in place, which includes a public education campaign to raise awareness of all phases of clinical trials. The campaign covers topics such as the aims of each trial phase, the rights of participants and the role of ethics committees to protect them, alongside practical information about the approval process and how to register.
“We have now launched our Clinical Trials Community Members database, a registry for healthy volunteers,” says Badriah Almutairi. “Around 400 people have registered in a short period, and some of them have been invited for screening to be enrolled in the current MERS-CoV vaccine trial.”
Further, Badriah Almutairi explains, they have set up a Twitter account (@KAIMRC_RF) specifically for the dissemination of accurate, easy-to-follow scientific information about clinical trials. They have also distributed printed media across the kingdom.
The ability to engage those who will ultimately benefit from trials can increase interest and participation. Perhaps, given the current global COVID19 pandemic, there may be an opportunity to help more people understand the importance of clinical trials, adds Huang.
“We will maintain an honest conversation with our society going forward,” says Badriah Almutairi. “We will communicate accurate information with the public, and we will share study results with all participants in simple, non-scientific language, so they can see the importance of their actions and difference they are making.”
KAIMRC is in the process of establishing a Subject Recruitment and Engagement office that is dedicated to keeping people engaged long-term. The office will answer all participants’ questions and invite them to workshops on pertinent topics like the subject’s rights, provide protocol rehearsals, and gather feedback to enhance the quality of engagement.
“We believe that educating people is the best way to ensure current and future participation,” says Adel Almutairi. “We have already received feedback from our volunteer registry stating that they feel they are helping humanity by helping to find cures and save lives.”
References
- Almutairi, A.F., Almutairi, B.M., Alturki, A.S., Adlan, A.A., Salam, M., Al-Jeraisy, M.I., & Balkhy, H.H. Public motives and willingness to participate in first-in-human clinical trials in Saudi Arabia: A new era in the making. Journal of Infection and Public Health 12 673-680 (2019) | article