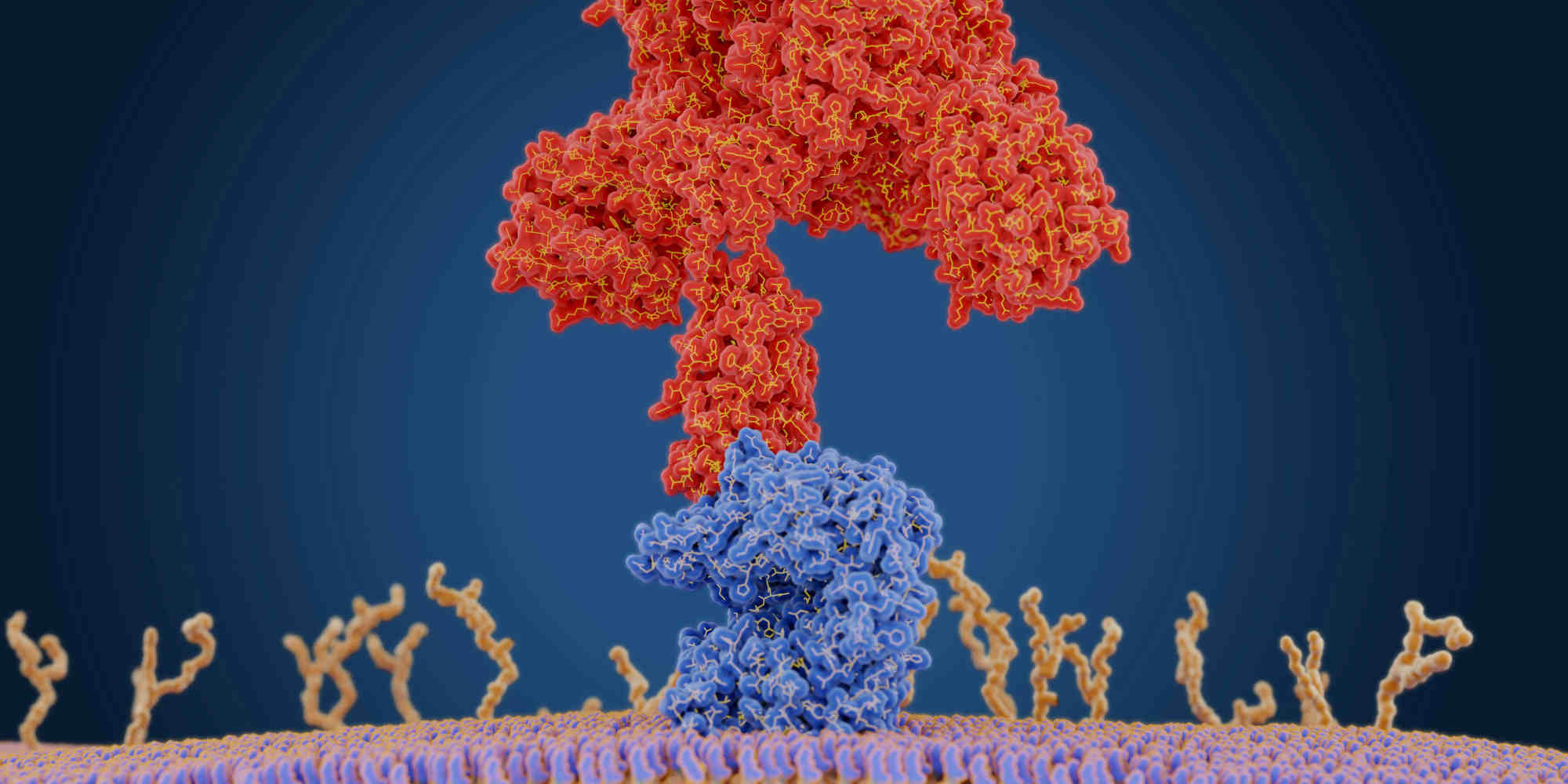
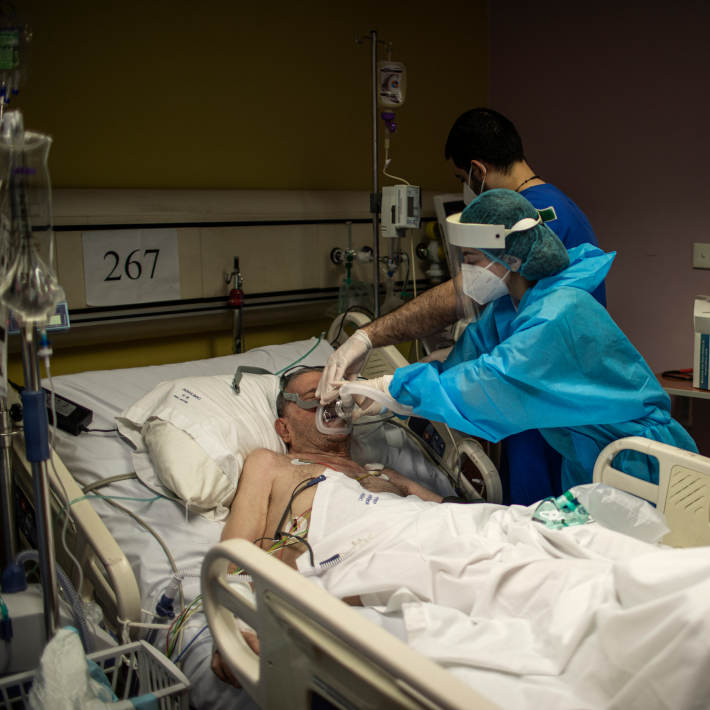
31 May 2021
Virologist and infectious disease researcher Naif Al-Harbi has been a central figure in KAIMRC’s coronavirus vaccine efforts. As the director of the vaccine development unit, Alharbi is leading KAIMRC’s phase I MERS vaccine clinical trial while also overseeing SARS-CoV-2-related research. He has spearheaded serostudies on COVID-19 to gauge immune response and to measure past and current disease risk in the Saudi population.
The Saudi Ministry of Health is overseeing vaccine acquisition and distribution. While there has been a lull in scheduling vaccinations due to Pfizer/BioNTech supply shortages, the situation is expected to improve in mid-February as the ministry tops up its stocks. The COVID-19 vaccine developed by AstraZeneca has also been approved for use in the kingdom, which should help relieve supply problems. In addition, new vaccination centres have been opened throughout Saudi Arabia to help meet the ministry’s goal and complete the vaccination of the remaining 26 million citizens by the end of 2021.
KAIMRC continues to focus on conducting and supporting vaccine research and testing more vaccines, in collaboration with the ministry, to expand the roster of shots.
At the end of 2020, Alharbi and a consortium of researchers published a survey of SARS-CoV-2 seroprevalence in healthcare and medical workers in Saudi Arabia. He and his colleagues are currently preparing to submit a national COVID-19 seroprevalence study after collecting upwards of 11,000 serum samples from across Saudi Arabia to survey national seroprevalence between June and November 2020.
KAIMRC Innovations spoke to Alharbi about this research, the kingdom’s preparedness for defence against the pandemic and KAIMRC’s unprecedented push towards expanding virus research.
Innovations: Many experts believe that KSA had a head start in responding to COVID-19 thanks to its previous experience with MERS. For instance, KAIMRC already had infrastructure such as vaccine development technology and diagnostics labs that were partly repurposed for SARS-CoV-2 research. Looking back at 2020, what crucial research did KAIMRC focus on in response to COVID-19?
Alhrabi: KAIMRC has a research programme on MERS-CoV that’s been going since 2015. This program has focused specifically on epidemiology, vaccine development, diagnostics, and therapeutic clinical trials. Recently, we published data from our RCT (The MIRACLE Trial) showing the benefit of a combo therapy of anti-viral agents and interferon beta 1B in treating moderate-to-severe MERS patients and reducing mortality. We also conducted a phase I clinical trial of the ChAdOx1 MERS vaccine, which was the first phase I trial in KSA. That helped us to be more prepared for COVID-19. The ChAdOx1 work we did with the University of Oxford helped with the launch and testing of their ChAdOx1nCoV-19 vaccine for COVID-19, which was further developed by AstraZeneca. We have also launched a number of clinical trials on COVID-19, including a trial to investigate the efficacy of Favibravir in treating COVID-19 (FACCT trial).
Innovations: KAIMRC is now collaborating with the Saudi Ministry of Health and the Saudi Food and Drug Administration (SFDA) to test nine vaccines, all currently in their third stage of clinical trials. Which vaccines are being considered at the moment, and what is their progress?
Alharbi: Our Vaccine Unit and Clinical Trial Unit, along with our strategy department and our leadership, have been in extensive negotiations with multiple international partners to accelerate the trials of several candidate COVID-19 vaccines. We have submitted at least three protocols to SFDA, one of which was for a phase III trial evaluating the efficacy of the COVID-19 vaccine CoronaVac, by Chinese biopharmaceutical company SinoVac.
After three cycles of extensive reviews, it was rejected by the SFDA. Early in March 2020, we started preparing a phase I/II trial submission for the ChAdOx1nCoV-19 vaccine together with Oxford, but at the time the rate of infection here wasn’t sufficient to carry out the trials; countries such as Brazil had many more cases, so ChAdOx1nCoV-19 could be evaluated more quickly there.
Innovations: How has KAIMRC handled such a tremendous research effort? Are new labs being set up at KAIMRC and its affiliate institutions and hospitals?
Alharbi: KAIMRC has established a Vaccine Unit and a Clinical Trial Unit, and they have been set up as the National Command Center for Clinical Trials (NCCCT). All that has helped in better preparedness. KAIMRC also has various labs focusing on genomics, microbial genetics, immunology, and cell biology. There’s also clinical research being conducted at the National Guard Hospitals, KAIMRC’s affiliated medical city. All of these research efforts have been moving together, in parallel.
Innovations: Before SARS-CoV-2 emerged, KAIMRC was hard in pursuit of a vaccine for MERS-CoV, cooperating with the University of Oxford and other top institutions. How has this quest been affected by the COVID-19 pandemic? Were efforts to find a MERS-CoV vaccine shelved or de-prioritized as a result?
Alharbi: We managed to complete the phase I trial, conducted entirely from December 2019 to November 2020, during the challenging times of the pandemic. Work on a MERS-CoV vaccine was not de-prioritised, but naturally it has been over-shadowed by the global and local focus on COVID-19 research, including at KAIMRC.
Innovations: What are some of the questions at the epicentre of research by coronavirus virologists and epidemiologists at KAIMRC?
Alharbi: As the whole world is entering the post-COVID-19 research era, KAIMRC is moving into research on the impact of COVID-19 and the long-term effectiveness of and response to the vaccines. We conducted collaborative work on neutralizing antibodies, as well as a serosurvey of SARS-CoV-2 among hospital workers early in the pandemic. The results, which were published late in 2020, showed that the public health measures, including vigilance in PPE and lockdown, worked to keep the rate of asymptomatic COVID-19 in health care workers at low levels. We are currently working to submit another national seroprevalence paper with more recent data.
This interview has been edited for length and clarity.
References
- Arabi, Y.M., et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials 21, 8 (2020). | article
- Hashem, A.M., et al. Early Humoral Response Correlates with Disease Severity and Outcomes in COVID-19 Patients. Viruses. 12, 1390 (2020). | article
- Alserehi H.A., et al. Seroprevalence of SARS-CoV-2 (COVID-19) among healthcare workers in Saudi Arabia: comparing case and control hospitals. Diagn Microbiol Infect Dis. 99,115273 (2020). | article
- FAvipiravir and HydroxyChloroquine Combination Therapy (FACCT) | article