11 February 2019
Most coronaviruses (CoV) preferentially target animals, but the recent emergence of pathogens from this family that cause potentially fatal disease in humans is the subject of much investigation. No medical treatments are available for severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), but new findings from George Gao and colleagues at the Chinese Academy of Sciences have revealed vulnerabilities in these viruses.
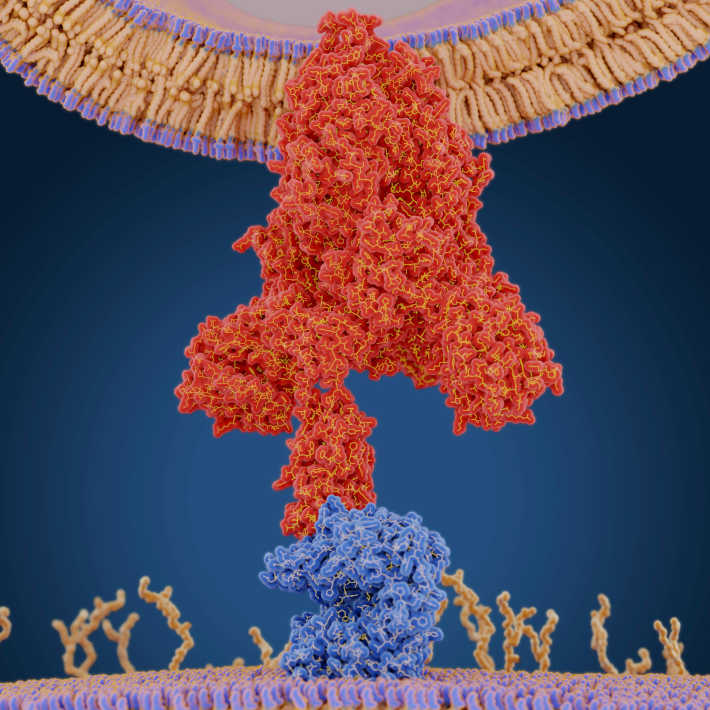
The SARS-CoV and MERSCoV viruses rely on a molecule called the S protein to penetrate host cells. In previous work, Gao’s team used X-ray crystallography to obtain a ‘snapshot’ of how a segment of this protein binds to its target receptor on cell surfaces. “This work elucidated the mechanism of MERS-CoV attachment to its host cell receptor, which is the first step of virus entry,” explains Yi Shi, a researcher on Gao’s team. “However, the subsequent steps — viral particle trafficking and membrane fusion — were poorly understood.”
Although crystallography worked well for studying the S protein’s receptor binding domain (RBD) in isolation, the full protein proved too floppy and dynamic to study with this technique. As an alternative, the researchers used a method called cryo-electron microscopy, which enabled them to study the MERS- and SARSCoV S proteins in a way that captures their structural diversity. They focused specifically on the ‘pre-fusion’ form of the protein, which assembles into complexes of three molecules and mediates the earliest stages of virus-host interaction.
“We noticed that both [MERS- and SARS-CoV S] proteins can be classified into several classes with different RBD conformations,” says Shi. In particular, they noticed two distinct RBD arrangements they termed ‘standing’ and ‘lying’ to reflect their orientation relative to the remainder of the S protein. By combining these data with their structural analysis of the interaction between RBD and host cell receptor proteins, they formulated a model for viruscell binding. Their data suggest that only ‘standing’ RBDs can bind to cellular receptors, an interaction that facilitates subsequent virus-cell fusion steps.
This analysis offers valuable starting points for fully reconstructing the process of viral entry, and could help researchers fight future outbreaks.
Because the standing RBD is highly exposed prior to receptor binding, it’s a promising drug target. “We are trying to develop neutralizing antibodies that target S protein for the treatment of emerging coronavirus infections,” says Shi. “Aided by structural analysis and functional characterization, we can identify the molecular basis of this neutralizing activity and improve their efficacy through molecular engineering.”
References
- Yuan, Y., Cao, D., Zhang, Y., Ma, J., Qi, J. et al. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat Commun. 8, 15092 (2017). | article