11 August 2016
After the notorious outbreak of severe acute respiratory syndrome (SARS) in 2003, another, potentially more deadly disease emerged. Middle East Respiratory Syndrome (MERS), also known as camel flu, has caused over 400 deaths since the first confirmed case in Saudi Arabia in 2012, and researchers are still searching for effective treatments. Now, a team at National Taiwan University in Taipei have revealed, for the first time, the exact structure of a crucial protein domain in the MERS virus that could be targeted by drugs.
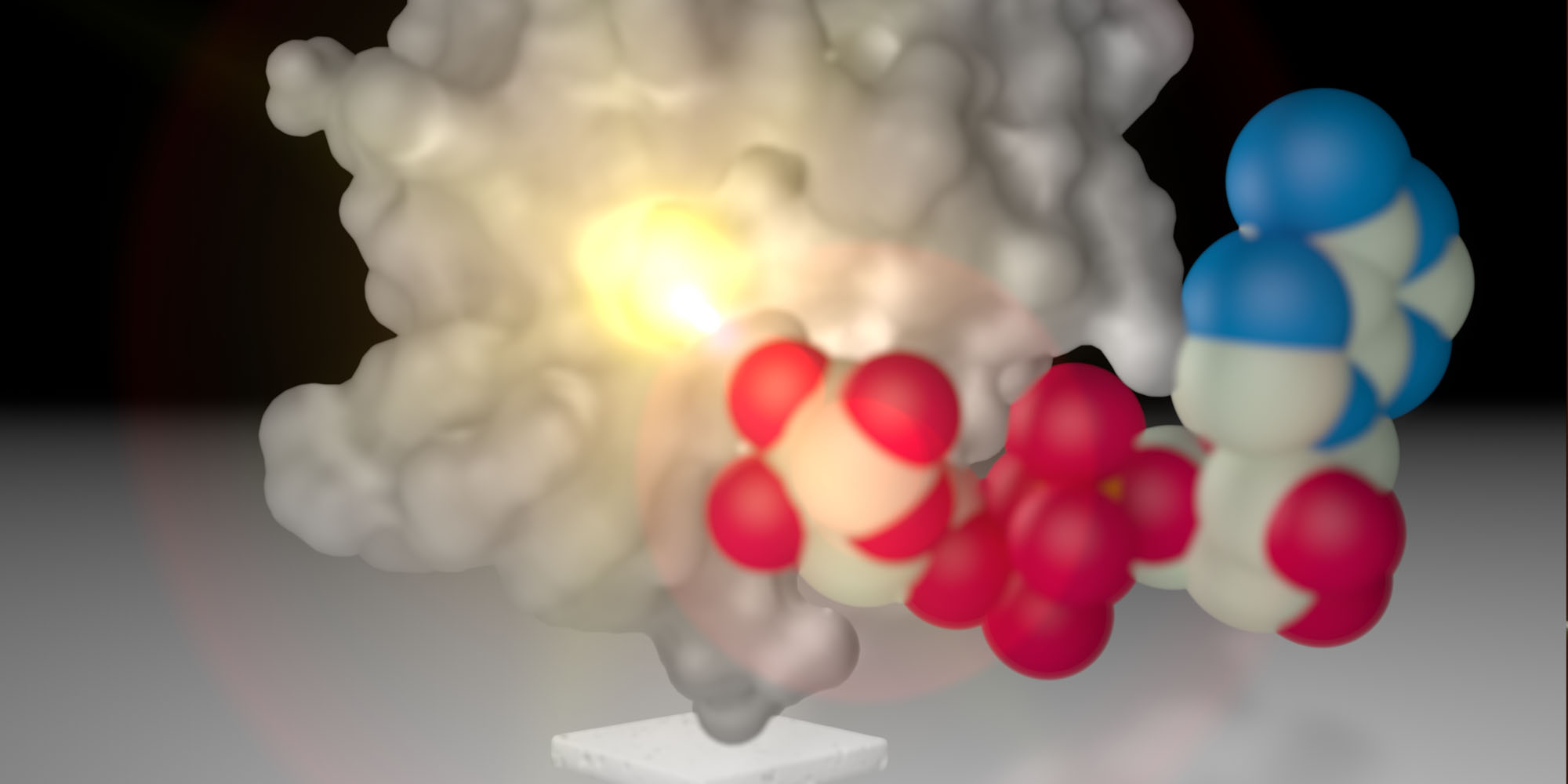
MERS is caused by a coronavirus that closely resembles the SARS virus, but it has a much higher mortality rate; 40% of MERS patients die compared to 10% for SARS. To find the reasons for this disparity, Chun-Hua Hsu and his team examined the non-structural proteins (NSPs) used by the coronavirus to rapidly replicate inside a host. Specifically, the team focused on the macro domain, a structure in NSPs that binds to the ester ADP-ribose to regulate cellular processes such as DNA repair, gene regulation and controlled cell death.
“Macro domain-containing proteins have diverse biological functions,” says Hsu. “However, there has been no conclusive answer about how viral macro domains function during infection. We found a macro domain on the MERS NSP3, and were curious about how its structure and function compares to macro domains from other coronaviruses.”
The researchers studied the macro domain structure using cutting-edge spectroscopy, fluorimetry, calorimetry and x-ray diffraction techniques. First, however, they had to crystallize their samples, which proved challenging.
“The MERS macro domain was quite unstable, and we found it difficult to grow crystals,” explains Hsu. “However, after we provided ADP-ribose, it resulted in crystals with good qualities that were suitable for diffraction data collection.”
The structural data showed the ADP-ribose molecule tightly bound in a crevice of the macro domain, where it is most likely stabilized by hydrogen bonds.
“We were surprised, because our data suggest that the MERS macro domain is more efficient at ADP-ribose binding than macro domains from other coronaviruses such as SARS,” says Hsu. “We speculate that this higher affinity for ADP-ribose allows the MERS macro domain to modulate host immunity during infection.”
Based on their findings, the researchers suggest that antiviral treatments designed to target the MERS macro domain could block viral replication or even clear the virus completely. They now plan to search databases of traditional Chinese herbal medicine in order to identify molecules that could inhibit the MERS macro domain.
References
- Cho, C.-C., Lin, M.-H., Chuang, C.-Y. & Hsu, C.-H. Macro Domain from Middle East Respiratory Syndrome Coronavirus (MERS-CoV) is an Efficient ADP-ribose Binding Module: Crystal Structure and Biochemical Studies. Journal of Biological Chemistry (2016) | article