11 August 2016
Researchers in the United Kingdom have unveiled the structure of the binding site for an anti-diabetic molecule called MK-0893, a potential target for diabetes therapies.
MK-0893 antagonizes the hormone glucagon, which is secreted by pancreatic cells and promotes glucose production when it binds to receptors on cells of several of the body’s organs.
The glucagon receptor belongs to a family of receptors that play a central role in metabolic and bone diseases as well as migraine and hormonal disorders. Several of these receptors work together to control glucose and insulin levels in the blood, but developing oral drugs that successfully regulate their function has proven difficult. In particular, small molecules, such as MK-0893, have been shown to reduce glucose levels in type 2 diabetes patients. However, in the absence of clear structural information, their modus operandi remains poorly understood; a major hurdle in structure-based drug design.
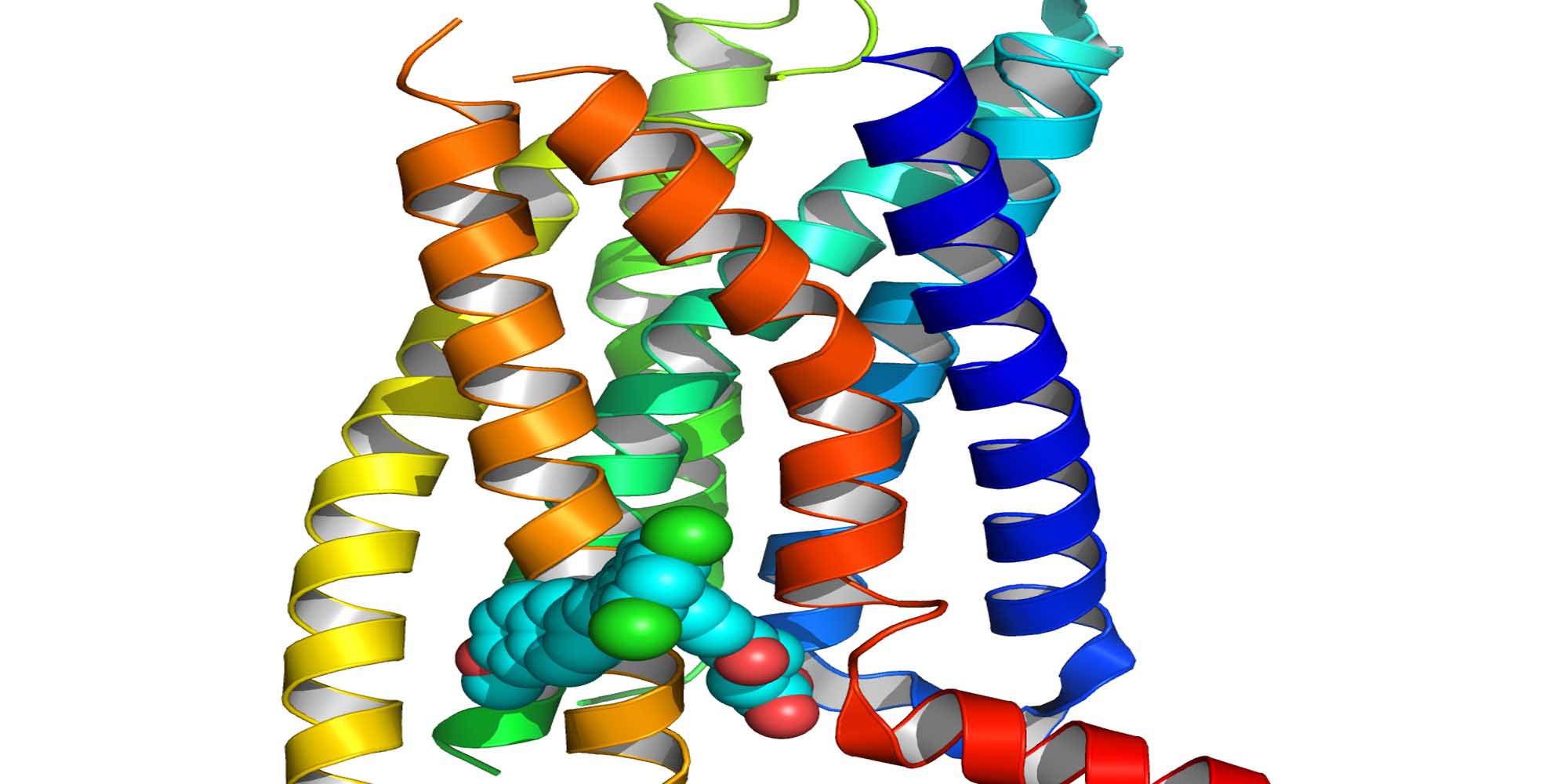
To tackle this problem, Fiona Marshall and colleagues from Heptares Therapeutics studied the structure of the receptor by crystallizing it in the presence of MK-0893. “Solving the X-ray structure of a protein allows you to see its exact three-dimensional shape and this can be used to conceive drugs that fit perfectly into the pocket on the receptor,” she says.
The researchers discovered that MK-0893 bound to a different site from the natural glucagon position on the receptor. According to Marshall, the structure suggests that the antagonist blocks the glucagon-mediated receptor activation by preventing the changes in receptor shape that usually occur when glucagon binds to it. “This is a new mechanism for this family of receptors,” she adds.
Furthermore, contrary to the team’s assumptions, the antagonist attached to the outside of the receptor instead of its inner core. This implies that the molecule reaches its binding site by passing through the lipid membrane that surrounds the cell and houses the receptor proteins. “This is in contrast to most drugs, which enter the receptor cavity directly from outside the cell,” Marshall says.
Marshall’s team is currently working on a rare disease called congenital hyperinsulinaemia that results in uncontrolled high levels of insulin, leading to extremely low blood glucose levels. She says that, using structural information, the researchers are working on drugs that target this disease by thwarting the signalling of the related receptor.
References
- Jazayeri, A. Doré, A. S. Lamb, D., Krishnamurthy, H. Stacey M. Southall, S. M. et al. Extra-helical binding site of a glucagon receptor antagonist.Nature (2016)|article