3 March 2019
Researchers in Canada have demonstrated how intermittent fasting can stimulate the metabolic system of mice to convert white fat tissue into energy-burning fat, thus reducing excess fat in the body. Their results could inform future treatments for type II diabetes and obesity.
“The link between metabolic diseases, unhealthy lifestyles and eating habits is clear,” says Hoon-Ki Sung at The Hospital for Sick Children and the University of Toronto, who led the international research team with his colleague Chi-chung Hui. “One possible intervention is intermittent fasting, which is already known to trigger a specific metabolic response.”
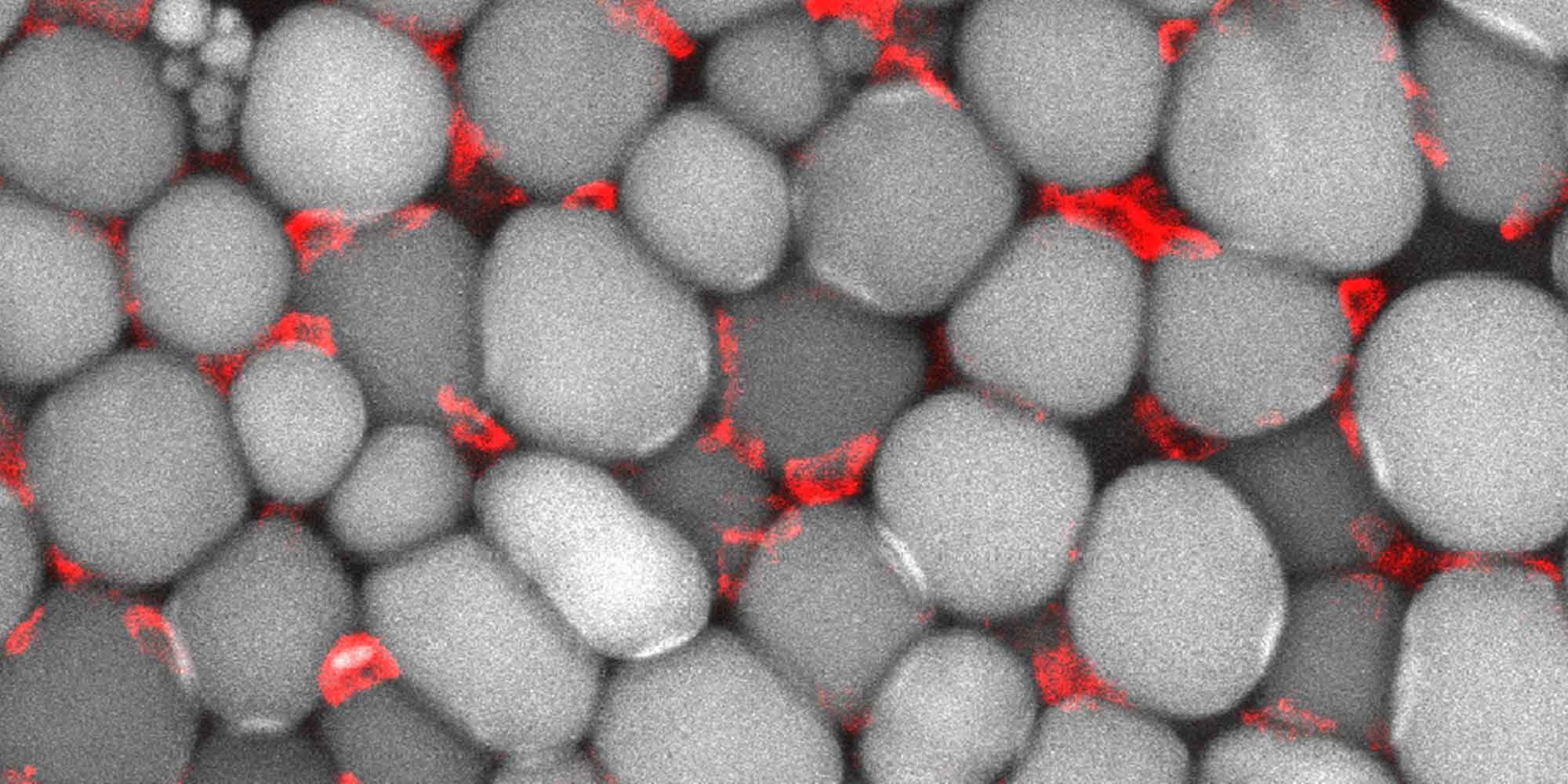
Fasting kickstarts a process that transforms white fat tissue, which stores energy and is present in excess in obese patients, into ‘beige’ fat, which fuels the body in lieu of food. Sung and Hui’s team wanted to find out exactly how this process works.
Previous studies on mice used a ‘one day fast/one day normal diet’ form of intermittent fasting, but it was unclear whether reduced calories were responsible for weight loss.
To address this, the researchers gave healthy, obese and diabetic mice a ‘one day fasting / two days normal eating’ regimen for 16 weeks, and compared the results with control groups. The animals in the intermittent fasting groups had the same overall calorie intake as control groups, fed with either a normal diet or 45% high fat diet, allowing accurate assessment of how fat tissues respond to fasting, regardless of calories.
All mice on the intermittent fasting diet had lower body weight than those on a non-fasting diet. This effect was most significant for mice on a high fat diet.
“We found that 2:1 intermittent fasting not only prevented obesity in mice, but also improved metabolic profiles of the diabetic animals by changing the quality of fat tissues,” says Sung. “The ‘stop-eating’ period stimulates anti-inflammatory immune cells found in fat tissues, and boosts levels of the VEGF gene, which is involved in blood vessel formation. These combine to transform white fat into energy-dissipating beige fat.”
Further experiments on fasting mice in which the VEGF gene was disabled showed that the browning of white fat did not occur, indicating that VEGF expression is a vital part of the process.
These findings suggest that the human metabolic condition may be significantly influenced by our daily eating cycle. “The next steps are to assess how our findings might be applied to the management and prevention of metabolic diseases,” says Sung.
References
- |Kim, K-H, Kim, Y.H., Son, J.E., Lee, J.H., Kim, S., et al. Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Research (2017).article