12 May 2019
The discovery of a protein’s contribution to the development of T-cells in the spleen and thymus could lead to new treatment strategies for immunodeficiency diseases.
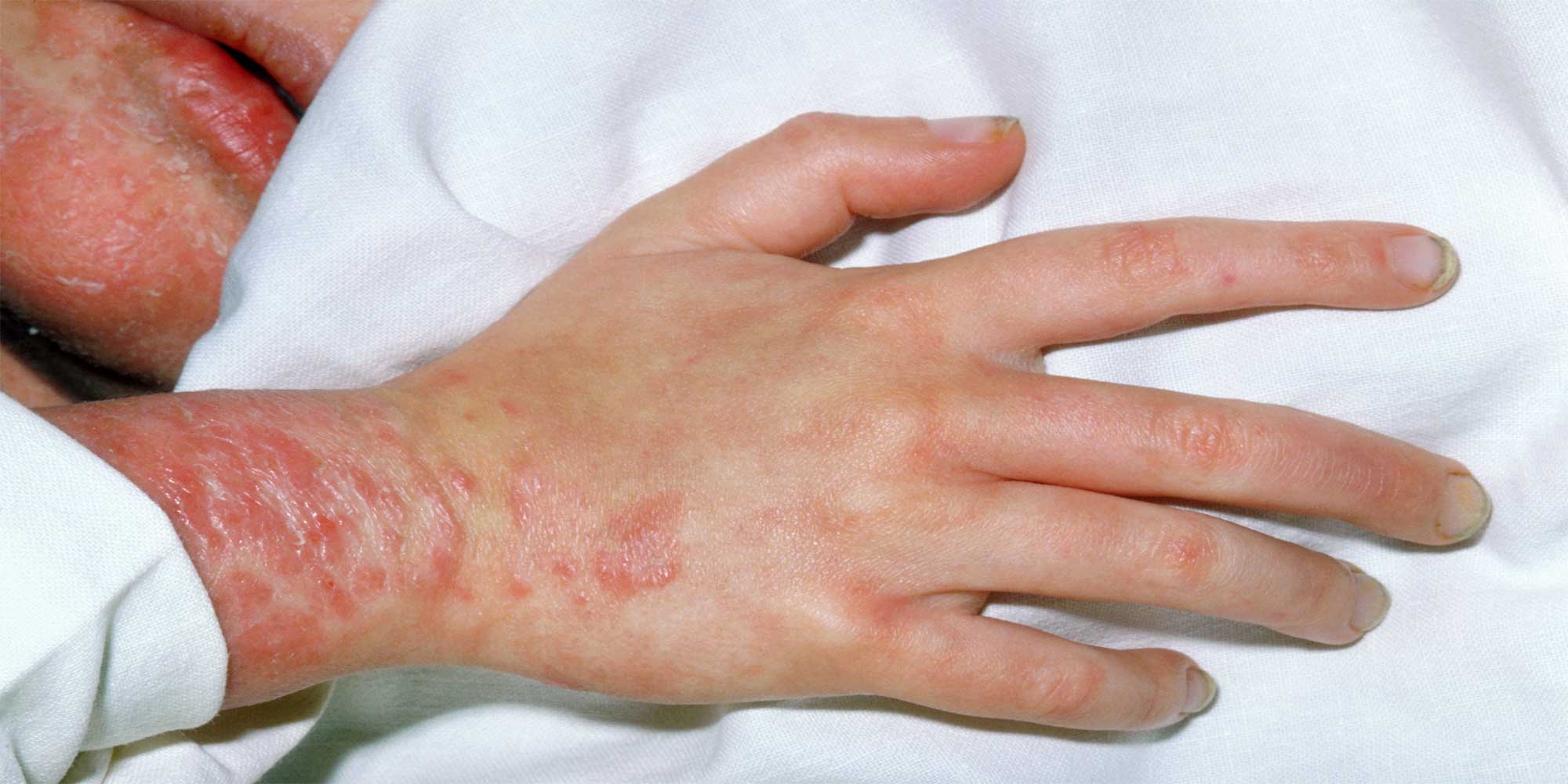
Despite its name, the Wiskott-Aldrich syndrome protein (WASp) is not only implicated in Wiskott-Aldrich syndrome, a rare genetic disease characterized by low platelet count, eczema and recurrent infections. Although mutations that abolish WASp expression can cause the namesake immunodeficiency disorder, other less disruptive DNA changes produce milder symptoms, such as low platelet counts, while mutations that put WASp activity into overdrive lead to low levels of infection-fighting white blood cells.
KAIMRC molecular geneticist Bader Almuzzaini and his colleagues set out to determine why different WASp mutations elicit diverse effects on the immune system. They focused on one population of immune cells in particular: helper T-cells, which serve as master regulators of immune defence.
The researchers used a method called ChIP-seq to find all the sites in the genome of mouse helper T-cells where WASp binds. They found that the protein interacted with both coding and non-coding stretches of DNA, and it was particularly prone to binding in or near genes involved in RNA synthesis.
Almuzzaini and his colleagues — including scientists from the Karolinska Institute in Sweden and Harvard Medical School in the United States — next wanted to identify the genes that are consistently regulated by WASp throughout the full process of T-cell maturation. They looked for sites where WASp bound to the genome in immature T-cells in the thymus and in more mature T-cells in the spleen. They pinpointed 15 genes and further investigated a few of them.
Experiments in genetically modified mouse models confirmed that one gene in particular — tcf12, which encodes a protein called T-cell factor 12 — relied on interactions with WASp to properly regulate the activity of other genes. Further studies showed that WASp plays a similar role for another important regulator called tcf1. These findings place WASp in close proximity with these two factors, showing that “WASp is an essential regulator for the maturation of T-cells during development,” says Almuzzaini.
It is perhaps not surprising then that aberrations in T-cell development are a hallmark of diseases linked to WASp mutations. However, it remains unclear how different WASp deficiencies lead to different clinical symptoms. “This needs further investigation,” Almuzzaini says. With that knowledge it should be possible to devise immune-modulating treatments that overcome the aberrations in T-cell function induced in WASP-associated diseases. “That,” says Almuzzaini, “is the ultimate goal.”
References
Kuznetsov, N. V., Almuzzaini, B., Kritikou, J. S., Baptista, M. A. P., Oliveira, M. M. S. et al. Nuclear Wiskott-Aldrich syndrome protein co-regulates T cell factor 1-mediated transcription in T cells. Genome Medicine 9, 91 (2017). | article