11 August 2016
A new, non-invasive method has been developed to monitor the effects of experimental gene therapies for cardiovascular disease and neurodegenerative disorders.
There is renewed optimism about research into new treatments based on replacing defective genes or inserting new ones. However, in some cases, progress is hampered by an inability to track the expression of introduced genes in experiments. Traditional histological approaches would be counterproductive because these would require removing samples of the very tissues the therapies are designed to protect.
Philip K. Liu, of Massachusetts General Hospital, Boston, and colleagues at Florida Atlantic University set out to develop a non-invasive method to monitor the expression of genes introduced to a brain after it has been damaged.
They began by observing how blocking the blood supply to the brains of mice for 60 minutes induced edema, or brain swelling, and general, severe weight loss. Also, 75% of the mice died within a week.
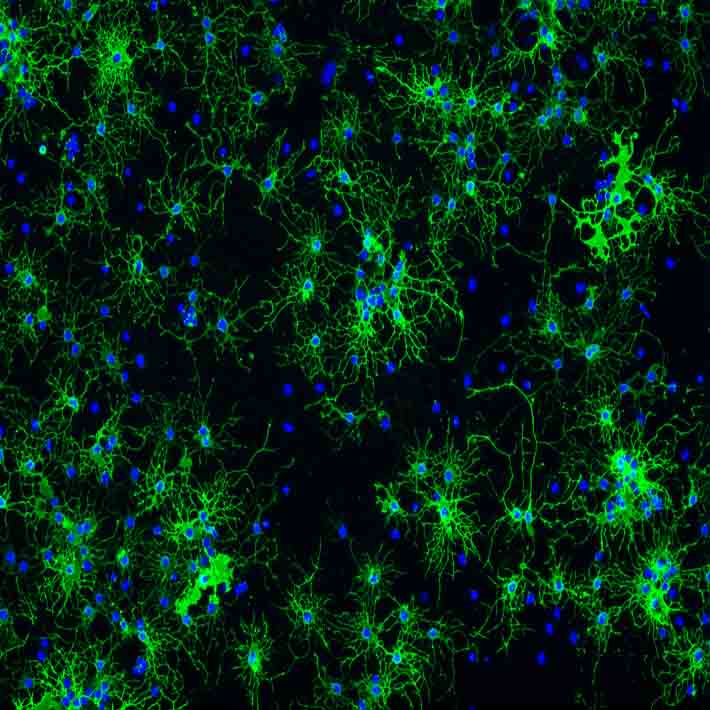
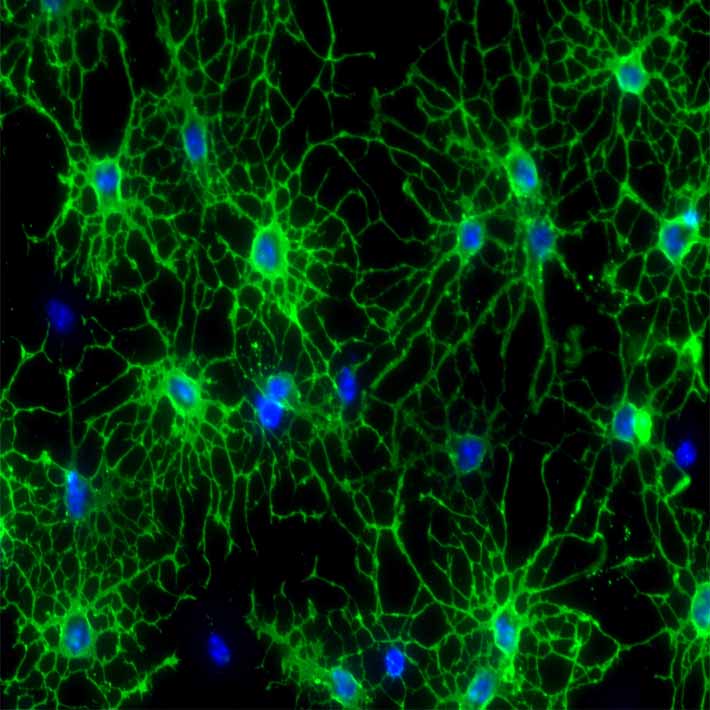
Brain-damaged mice were then given eye drops containing a virus modified to carry genes that encode human granulocyte colony-stimulating factor (hG-CSF). This is a protein that triggers the release of certain white blood cells and stem cells into the bloodstream.
In animal studies, G-CSF has been shown to provide protection in response to brain damage by increasing blood vessel growth, protecting neurons, reversing memory loss and reducing excess in beta amyloid deposits – which have been linked to Alzheimer’s disease and stroke.
The survival rate among the brain-damaged mice given the gene therapy eye drops was between 33 and 100%, depending on how soon after oxygen deprivation they were treated, compared to around 25% in a placebo group.
“Growth factors have been shown to help reverse brain damage, but we were still surprised at how effective it was,“ says Liu.
The researchers then attached magnetic resonance contrast agent nanoparticles to DNA capable of targeting the messenger RNA that expresses hG-CSF, and used MRI scans to successfully track expression from nerve cells in the damaged brains of living mice.
The technique has the potential to provide a new targeted way to monitor the expression of introduced genes for scientists working on therapies for conditions including heart attack, stroke, Alzheimer’s and Parkinson’s disease.
References
-
Ren, Y., Chen, Y. I., Liu, C. H., Chen, P-C., Liu, P. K. et al. Noninvasive tracking of grene transcript and neuroprotection after gene therapy. Gene Therapy (2016). | article