22 July 2020
Genetic testing of newborns can give doctors essential information about hereditary disorders that might be mitigated or even prevented if identified and treated early. KAIMRC’s Majid Alfadhel and his colleagues demonstrated the feasibility of detecting mutations associated with a high-risk vitamin deficiency, although its incidence may be too low to justify widespread screening.
Children born with biotin-thiamine-responsive basal ganglia disease (BTBGD) carry mutations in both copies of a gene known as SLC19A3. This gene encodes a transporter protein that manages the cellular uptake of thiamine, also known as vitamin B1. The resulting deficiency leads to neurological symptoms such as seizures and cognitive and motor deficiency, which can become severe and even fatal over time. However, Alfadhel and colleagues found that prompt treatment with thiamine and another B vitamin, biotin, can prevent such outcomes and help healthy development.
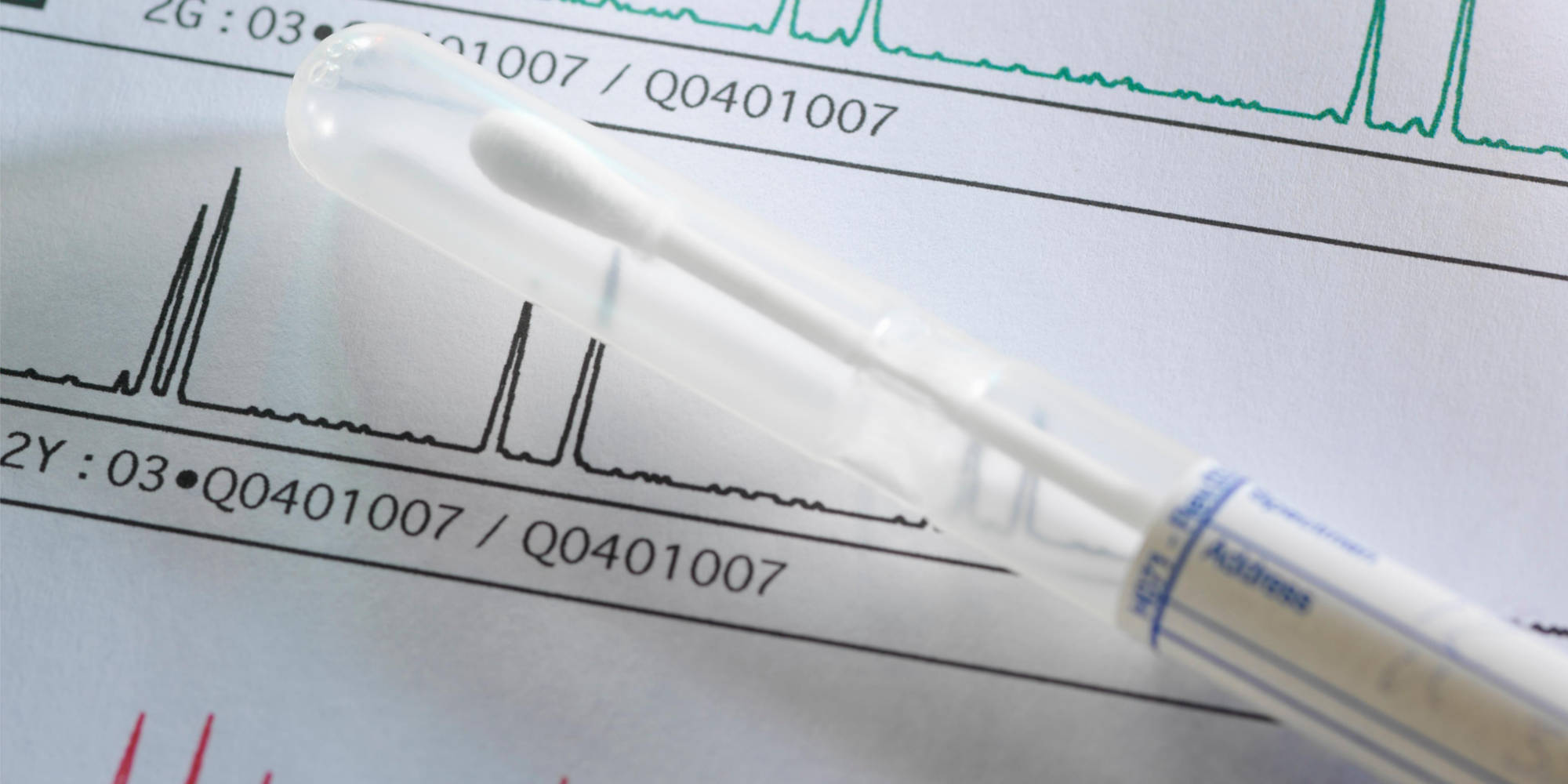
Although generally rare, 50% of all reported cases of BTBGD came from the Saudi population. “Since we have the largest cohort of patients in the world, and this is a treatable condition, we decided to do a pilot study to know the incidence of this disease in Saudi Arabia,” said Alfadhel. In order to get a better handle on the disease’s prevalence in the country, Alfadhel and colleagues performed genetic analysis of the SLC19A3 gene in 3,000 healthy babies born at two Riyadh-based hospitals over a two-year period.
Six of these infants had a single copy of the disease-causing mutation, making them asymptomatic carriers. None of these were born into families with a reported history of this condition, and the researchers subsequently recommended that other relatives of the affected children also undergo genetic screening to assess their own risk of transmitting the mutation to their own children in the future.
These results revealed a 1 in 500 risk of carrying this particular SLC19A3 mutation, leading the researchers to estimate that 1 in a million children are born with BTBGD. Although 37 other BTBGD-associated mutations have been described to date in the literature, none of these variants was observed in this particular cohort.
The Saudi newborn screening panel currently looks for 17 hereditary metabolic and endocrine disorders, encompassing conditions that occur with incidences ranging from roughly 1 in 7,000 newborns to just under 1 in 200,000. Given the apparent relative rarity of BTBGD, Alfadhel and colleagues advise against adding it to the panel at this time. “However, we certainly need to screen high-risk groups like first degree relatives, and do premarital screening for families screening from this disorder,” he says. They also note that the current cohort was limited in size, and suggest that additional studies in a larger population should be conducted before making a final recommendation.
References
- Alfadhel, M., Umair, M., Almuzzaini, B., Alsaif, S., AlMohaimeed, S.A. et al. Targeted SLC19A3 gene sequencing of 3000 Sauid newborn: a pilot study toward newborn screening. Ann. Clin. Trans. Neurol. | article