27 May 2021
Mutation of a gene linked with primary congenital glaucoma (PCG) alters the performance of cells that surround, protect and maintain the optic nerve. The progression of glaucoma is not completely understood, but these findings shed some light on the molecular mechanisms behind the disease and its complications.
PCG affects children younger than four and is responsible for 5% of childhood blindness worldwide. A mutation in the CYP1B1 gene is responsible for 63% of PCG cases in the western region of Saudi Arabia. However, how this mutation results in damage to the optic nerve is still unclear.
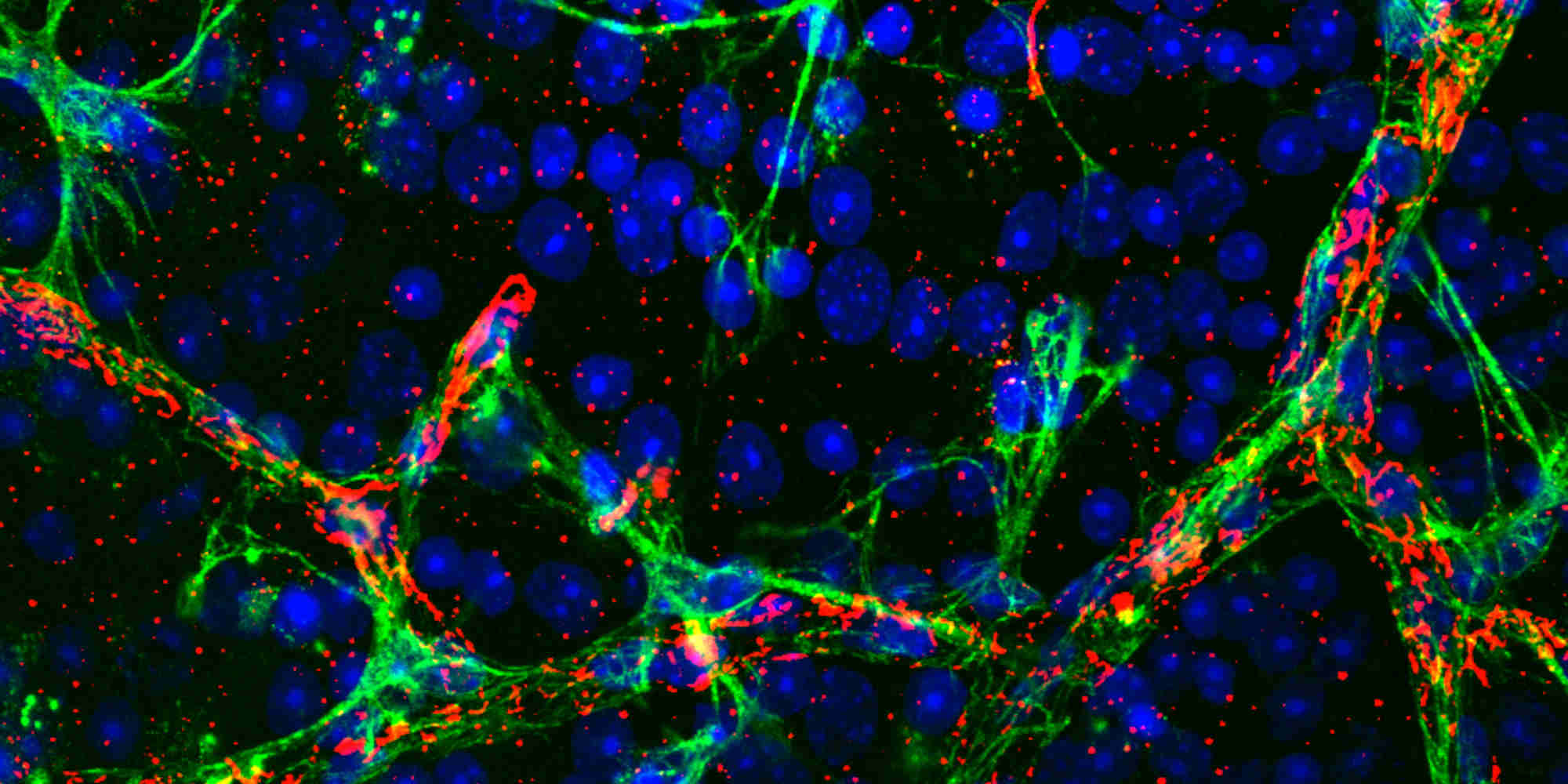
Researchers at KAIMRC, King Saud Bin Abdulaziz University for Health Sciences and King Saud University used CRISPR to introduce the mutation into cultured microglia, astrocytes, and mesenchymal stem cells isolated from rats. They found that the mutation has particularly severe consequences in microglia, which are known to play a vital role in the immune defence of the central nervous system.
The growth of microglia dropped by around 60% three days after induction of the mutation. In addition, many of the mutated microglia died prematurely, leading to an elevated inflammatory response. Together with an increase in cellular stress and a decline in mesenchymal stem cells, this could cause damage to the extracellular matrix, which provides structural and biochemical support to the optic nerve, as well as the trabecular meshwork, which controls the pressure inside the eye.
“Microglia keep surprising scientists with their activities. I would like to understand whether keeping them alive helps treatment or medical management,” says KAIMRC’s biomedical scientist, Bahauddeen M. Alrfaei, leading author of this study. In addition, monitoring microglia in glaucoma animal models will lead to new and better therapies.”
The researchers also reported an increase in the expression of several pro-inflammatory proteins. In particular, MMP-2 production was increased in mutated astrocytes, cells which are involved in the maintenance and survival of neurons. This could further damage the extracellular matrix surrounding the optic nerve.
Altogether, these findings show that CYP1B1 mutation may disrupt eye development through the loss of microglia, degradation of the extracellular matrix, and increased cellular stress. The researchers suggest that the resulting inflammation might explain why some types of eye surgery for glaucoma patients fail. “We hope that this and future studies will help us to understand whether the inflammation in childhood glaucoma is preventable via anti-inflammatory treatments or other non-invasive procedures,” says Alrfaei.
References
- Alghamdi, A., et al. The loss of microglia activities facilitates glaucoma progression in association with CYP1B1 gene mutation (p. Gly61Glu). PLOS ONE 15.11 (2020): e0241902. | article