30 September 2021
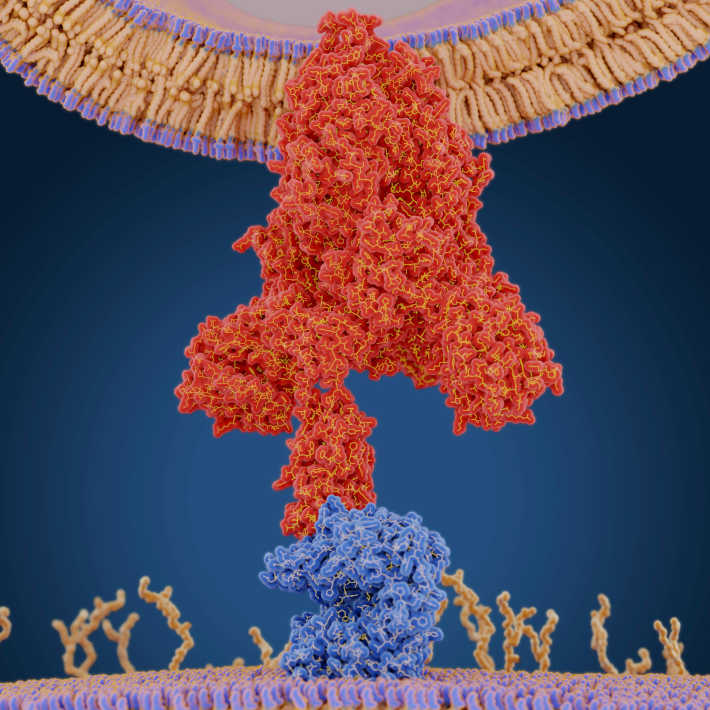
The Middle East respiratory syndrome coronavirus (MERS-CoV) currently poses a relatively minor threat to human health, but more dangerous strains could emerge through natural mutation. Researchers at Regeneron Pharmaceuticals have demonstrated that treatment with a pair of virus-binding antibodies could confer potent protection against infection with MERS-CoV, even when administered after exposure to the virus.
MERS-CoV has about 34% mortality rate in humans, but the spread of this virus has been limited, with only about 2,600 cases reported, almost all in the Arabian Peninsula. But the virus continues to circulate widely in camels throughout the Middle East, and as COVID-19 has demonstrated, new coronavirus variants can emerge that readily jump from animals to humans. “All coronaviruses mutate as they replicate, and the resulting mutant virus always has the chance of gaining increased fitness,” says Matthew Frieman, a microbiologist at the University of Maryland.
Our bodies can naturally fend off infection by producing virus-specific antibodies, and Regeneron has been exploring the possibility of bolstering this protection through treatment with engineered antiviral antibodies. Regeneron researchers, in collaboration with Frieman’s lab, developed a pair of promising antibodies for MERS-CoV in 2015, and the two groups recently teamed up again to test how well these can control infection.
They first tested in the preclinical study the antibody combination in a ‘humanized’ mouse model that is susceptible to MERS-CoV infection. Whereas virus levels surged within two days post-infection in control animals, pre-treatment with the two antibodies effectively quashed viral replication in the lungs. Importantly, the antibodies were equally effective when administered one day after infection. “This gives some confidence that treatment in humans before infection isn’t required,” says Frieman, adding that this would be valuable in terms of enabling prompt intervention when the earliest signs of disease appear. The researchers also conducted an initial safety test in healthy adults subjects in the following phase 1 study, and demonstrated that the antibody cocktail was stable in the bloodstream and generally well tolerated.
Further testing in MERS-endemic regions will be needed to determine the protective power of these antibodies in humans, but Frieman is encouraged by these preliminary results. “We should be preparing for the time when MERS-CoV evolves to a more problematic virus and have therapies ready,” he says. And as the scientific community continues to improve its understanding of the immune response to SARS-CoV-2 that determines the ultimate severity of infection, Frieman sees exciting potential in working towards developing broadly-protective ‘pan-coronavirus’ antibodies.
References
- Sivapalasingam, S. et al. Human Monoclonal Antibody Cocktail for the Treatment or Prophylaxis of Middle East Respiratory Syndrome Coronavirus. J. Infect. Dis. Published online 28 January 2021. | article