28 June 2021
In the opening keynote of the COVID-19 Vaccine Forum hosted by KAIMRC in November 2020, Professor Bali Pulendran of Stanford University declared that he was “in awe” of the rapid pace at which COVID-19 vaccines have been developed. He explained how the many phases of vaccine research, trials, regulatory approval and large-scale manufacturing had often previously taken 10 years or more. But in the case of COVID-19, the first large scale vaccinations began on 8 December 2020, just under a year since the first case of the disease was reported to the World Health Organization.
At the KAIMRC Forum, Professor Adrian Hill of the University of Oxford summarised the key factors behind the astonishing success of what he called “the grandest global experiment in vaccine technology ever undertaken.” He emphasised the unprecedented collaboration involving thousands of different specialists worldwide, all focusing their multidisciplinary attention on one target. They worked at record speed to sequence the viral genome, analyse its structure and explore the options for vaccine development.
Hill then turned to the role of unprecedented levels of funding from governments and other bodies, saying that for the first time in his career almost “unlimited funding” has been available. He also talked of a new willingness by regulators to work much faster than normal, which he views as one of the major lessons to be learned for vaccine development in the future. Hill explained that there is no single global regulatory body for vaccines, which would simplify the currently cumbersome process of dealing with regulators in different countries who often take different views on the same clinical data.
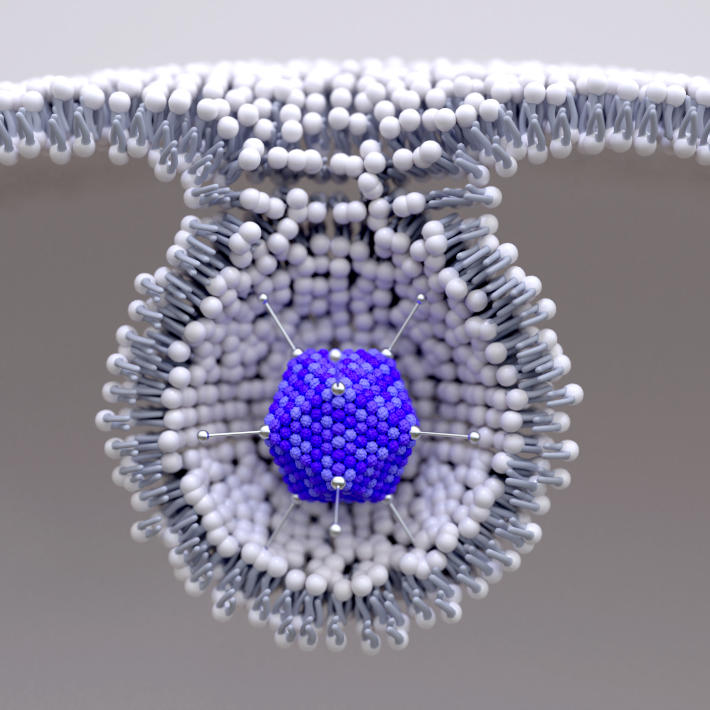
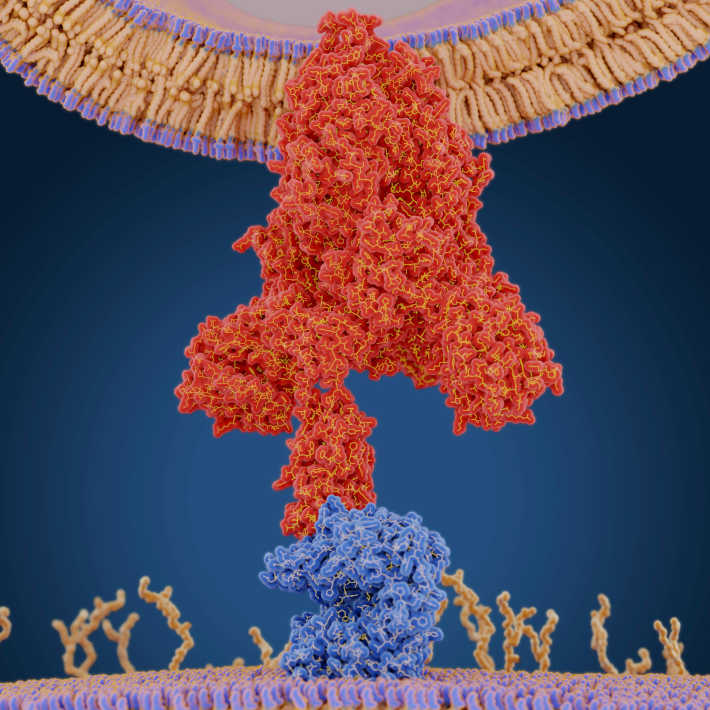
The crucial basic research itself was accelerated by researchers being well primed by existing work and by the novel nature of the first vaccines. Rather than the traditional approach of administering viral proteins directly, these are based on RNA or DNA that instructs the body’s cells to manufacture a key immunogenic part of the viral coat’s spike protein. Researchers working on these vaccines say they could be developed quickly in part because we already had the technology to rapidly and routinely synthesize RNA or DNA of a desired sequence. RNA and DNA vaccines have never previously been licenced for use in humans, although they have been used in veterinary applications and in many laboratory tests on animals.
The Pfizer/BioNTech and Moderna vaccines use synthetic single-stranded messenger RNA (mRNA) molecules that contain the genetic instructions to directly induce the body’s cells to make the proteins that stimulate immunity. The Oxford-AstraZeneca vaccine achieves a similar outcome using double-stranded DNA, which is transcribed into mRNA by the normal metabolic activity within a cell. The Oxford team point out that DNA is more robust than mRNA and the adenovirus’s tough protein coat also helps protect the genetic material inside, which makes their vaccine stable at significantly higher temperatures than mRNA-based vaccines —a significant benefit.
The researchers behind the Oxford-AstraZeneca vaccine credit the “head start” they got through their many years of research on DNA vaccines as a key factor behind their rapid response. Professor Sarah Gilbert, who designed the vaccine, explained to the KAIMRC Forum that the COVID-19 vaccine delivers DNA using a system which had already been developed in their laboratory. A harmless, modified adenovirus carries the DNA in their vaccine. “We had used this to make many different vaccines in the past,” Gilbert said, some of which were already in phase 1 clinical trials.
All that is required to adapt this versatile system to a new disease is to change the DNA sequence being delivered. Together with an intensive research effort, this head start made it possible for the vaccine to move into clinical trials in April 2020, a mere 104 days after the researchers first received the genetic sequence of the SARS-CoV-2 virus responsible for COVID-19.
This feature was also a key factor in accelerating the production of the Pfizer/BioNTech and Moderna vaccines. Pfizer, which made the first COVID-19 vaccine to get regulatory approval, emphasized the significance of their partnership with German company BioNTech in meeting the challenge so quickly. They say this brought together BioNTech’s specialist expertise in developing mRNA vaccines with Pfizer’s more broad expertise in vaccine technology, regulatory issues, and manufacturing and distribution.
Most of the researchers placed particular emphasis on the unusually quick efforts of regulatory bodies worldwide to assess the results of fast-tracked clinical trials, in many cases, granting Emergency Use Authorizations for the earliest vaccines. These allow the vaccine to be used based on preliminary data provided that further surveys of efficacy follow as vaccination gets underway.
The phases of vaccine trials normally proceed consecutively and usually take several years. In the case of COVID-19, the massive funding available and the urgency of the situation made it possible to overlap some of the clinical trial phases. The researchers also pointed out that the vast number of infections worldwide made it easier and faster to run trials with many participants in a shorter time while still getting sufficient evidence to demonstrate efficacy.
“Vaccines are good for pandemics, but pandemics are good for vaccines,” Adrian Hill says. The fact that COVID-19 rapidly became a pandemic helped get vaccines quickly developed and tested. For example, the Oxford-AstraZeneca team report that their vaccine “will have been tested on almost five times as many volunteers as is usually required for licensing a vaccine.”
Hill also calls for regulators worldwide to come together to simplify and accelerate the regulatory process, which currently involves multiple national regulatory bodies and the ensuing complexities. According to him, this is the major change needed to improve vaccine development in the future.
The key players in academia and industry generally agree that the experience of developing COVID-19 vaccines is likely to significantly improve the process in the future. If the new and quicker technology of RNA and DNA vaccines proves effective in the long term, it could accelerate vaccine development overall. The vast funding thrown at the COVID-19 challenge is unlikely to be available again, but the lesson that regulatory bottlenecks can be overcome may also bear fruit. If these lessons are learned, “developing new vaccines in three years instead of ten years would become a bit more realistic,” says Hill.
References
- | article
 <
<