27 September 2021
AZD1222, developed by the University of Oxford and licenced to AstraZeneca, was among the first vaccines created to protect against COVID-19. A non-replicating viral vector vaccine, it was approved for emergency use in several countries but later put on hold because of links with the formation of dangerous blood clots. While the clinical trials and authorization procedures have received significant press coverage, less has been said about the trailblazing technology that underpins AZD1222 – a modified chimpanzee adenovirus called ChAdOx1.
Viral delivery
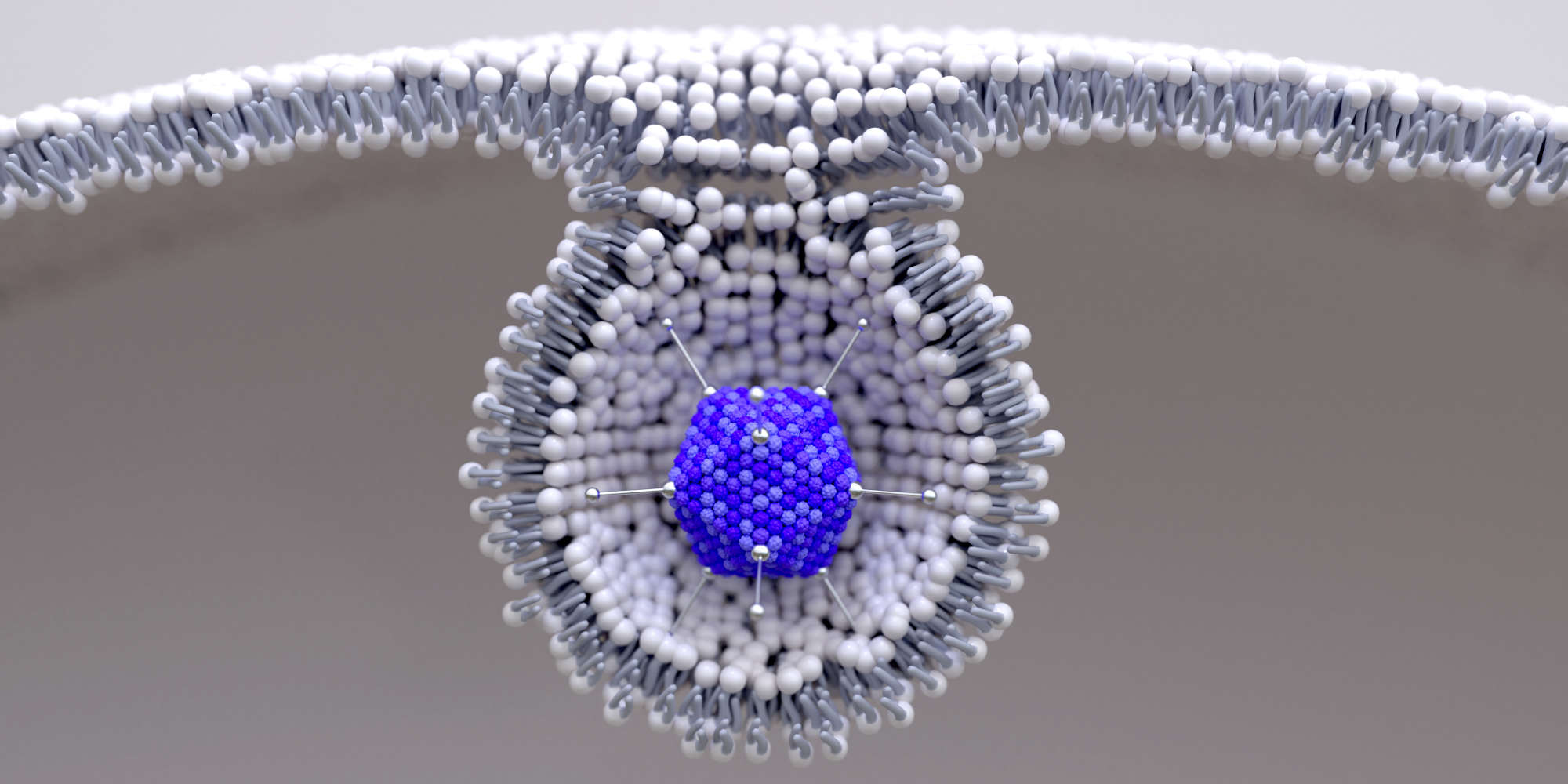
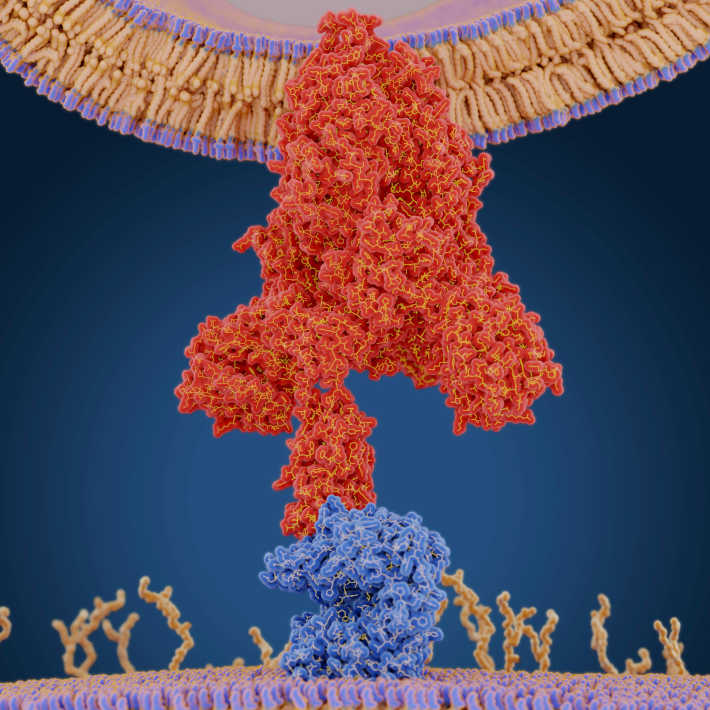
Viruses hijack infected cells and force them to produce copies of the virus itself. Scientists have long leveraged this ability to create non-replicating ‘viral vector’ vaccines, in which a virus is genetically engineered to reduce or eliminate pathogenicity but still prompt cells to produce the immunogenic proteins of another virus. This method exposes a host’s immune system to specific proteins from a target virus but not the virus itself, enabling the host to safely build immunity . In the case of AZD1222, this protein is the spike protein of SARS-CoV-2, the virus that causes COVID-19.
In 2012, researchers at the University of Oxford created ChAdOx1, a virus vector vaccine based on a modified simian adenovirus. The researchers chose a simian adenovirus because “if you use a human adenovirus to immunise humans, you run into the problem of pre-existing immunity,” says Sarah Gilbert, professor of vaccinology of the University of Oxford and co-founder of the spin-off Vaccitech, which patented the ChAdOx1 technology. Following successful collaborations with other institutions using other simian adenoviruses, Gilbert says “we wanted our own. We managed to acquire a chimpanzee virus that had been described in the literature, and then a student in the lab converted it to a vaccine vector” – and ChAdOx1 was born.
To create the platform, Gilbert’s team modified the viral genome to remove its ability to replicate and to optimize its manufacture. They can then insert the gene encoding a desired viral protein. The end result is an injectable solution that infects cells around the injection site and causes very high expression of the target protein. In the case of AZD1222, “something like 80% of the protein expressed from the adenovirus is spike protein,” says Gilbert.
The host immune system learns to recognize and respond to the expressed protein, but because the virus can’t create new copies of itself, the short-lived infection doesn’t proliferate around the body and cause disease. This makes the platform very safe to use, even in people with a compromised immune system, says Gilbert. “It's as if you have a coronavirus infection in your arm, but it's not coronavirus, it's the adenovirus and it can't replicate.”
ChAdOx1 as platform rather than product
Gilbert describes the modified simian adenovirus as a “true platform technology” which can be modified to cause the expression of different viral proteins without any alterations to manufacturing or safety. Oxford researchers are using their platform to investigate vaccines for many diseases, such as tuberculosis, rabies, MERS, Dengue, and Zika virus.
Ahmed Salman, a senior vaccinologist and immunologist at the University of Oxford, adds that ChAdOx1-based vaccines are easy to produce and stable once made, saying that large quantities can be produced “at a really low cost in a short amount of time.” The platform is also very effective at inducing a strong response from both B cells and T cells, whereas competing technologies often generate a skewed response.
At the virtual KAIMRC conference “COVID-19 Vaccines: Global Challenges & Prospects Forum,” Gilbert described how her team managed to reach their first trials in humans just 103 days after receiving the genetic sequence of the spike protein.
Vaccine sceptics believe that such rapid development means that these therapeutics must be poorly understood or poorly tested. Salman argues that vaccine research is normally hampered by slow bureaucratic processes and time wasted waiting for grants, approvals, and publication – all while people are suffering from a disease. With COVID-19, he says, we have a “good example” that things can be more streamlined. “I work on malaria, which doesn’t get [the same attention as COVID-19]. We’ll be in clinical trials in a few weeks, but we started research more than seven years ago.”
Furthermore, vaccines such as AZD1222 weren’t created from scratch for COVID-19; rather, they build on decades of existing research. Scientists also did not have to face the pandemic blindly – it was anticipated. In 2016, Salman says, the University of Oxford started its “Pandemic X” project. Scientists prepared plans based on anticipating what diseases might cause a global pandemic, what therapeutics might help, and what infrastructure should be put to work to abate disaster. Salman adds that there have been many regional epidemics since 2000, such as swine flu, avian flu, SARS, MERS, Zika, and Ebola, which have offered lessons in preparedness. In this context, it’s clear that the global research community needs to continue to prepare for what may come next.
Building for the future
For Gilbert and Salman, work hasn’t stopped with the rollout of the Oxford/AstraZeneca vaccine. “We’re really busy,” says Gilbert, adding that the trials are ongoing. The researchers will follow up with trial participants six months and one year after their second dose to collect blood samples and generate long-term data. Gilbert and her team have extended their studies to include an HIV-positive cohort in the UK and South Africa. However, many of these trials have been halted because of fears that the vaccine can cause dangerous blood clots in rare cases.
The concerns appeared after the vaccine’s rollout, when several countries reported that it appears to be linked with dangerous blood clots in very rare cases. Despite the rarity, this led some regulators to temporarily suspend use of the vaccine and later to authorize its use only by some groups, such as older people. Overall, the consensus among health officials is that the benefits still outweigh the risks, but research is being done to under the mechanism behind the clotting in the hope that this will make it possible to reduce the risks further, as well as helping guide the evaluation of other vaccines.
“We’ve got approval in 15 countries so far, but there are other regulators still making their assessments and sending through questions that we need to answer very quickly,” says Gilbert. “And once there’s any time to do anything else, I’ve got all the other vaccine projects that I should have been working on last year.”
References
- | article