4 June 2017
Sequencing the entire protein-coding portion of the genome in people with unexplained intellectual development disorders can offer diagnoses and new treatment options for a large number of people.
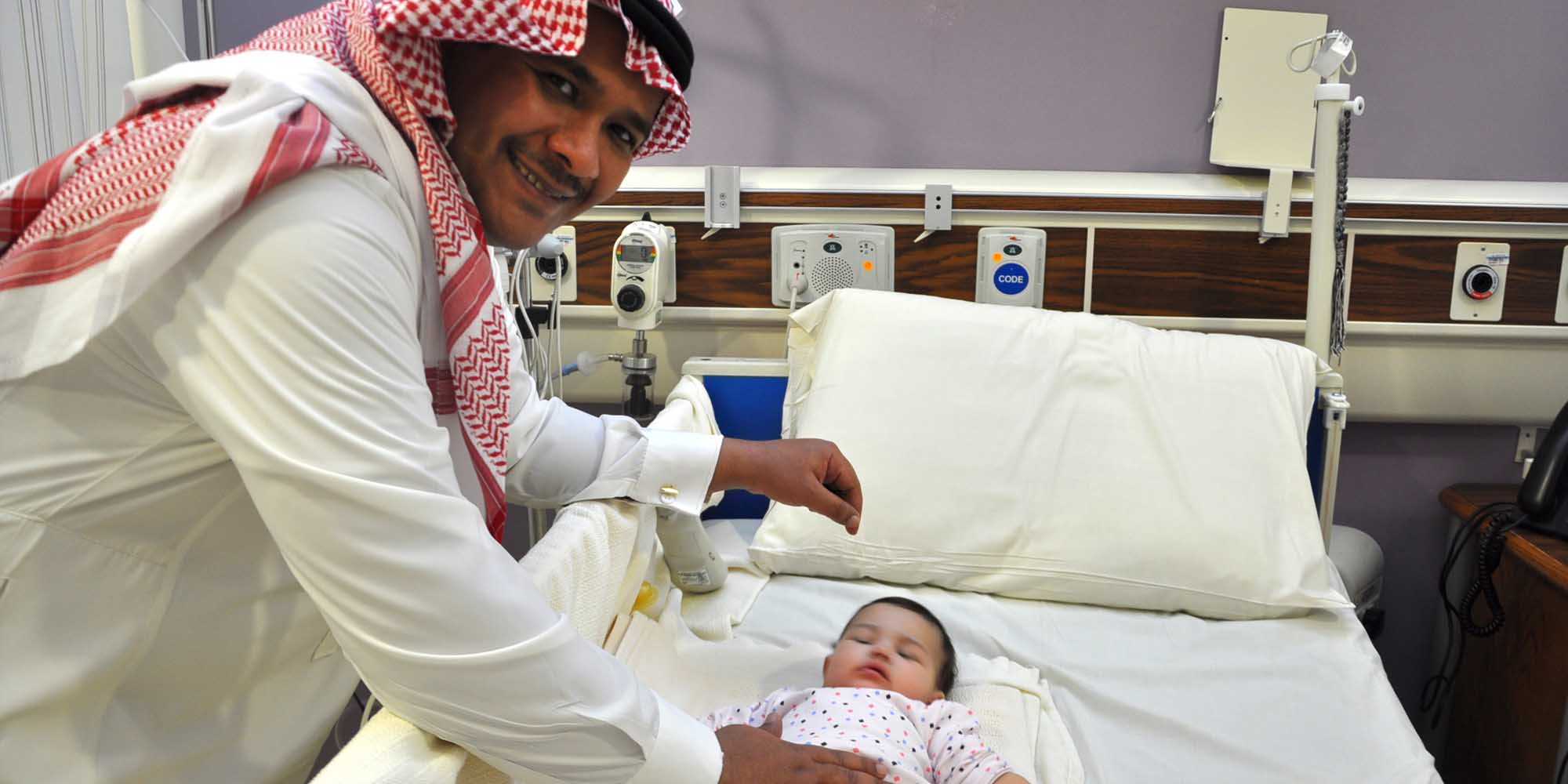
A Canadian-led team, which included KAIMRCpaediatric geneticist Majid Alfadhel, conducted extensive clinical evaluations and DNA sequencing on 37 children and four adults with unexplained intellectual development disorder, a condition that affects an estimated 3% of the population worldwide. This approach yielded a genetic diagnosis for 28 of the patients, including a four-year-old Saudi girl with recurrent breathing problems, skin pigmentation abnormalities and fat deposits in her liver. Sequencing the 1% of this child’s genome that codes for proteins revealed that mutations in a gene called H6PD, which encodes an enzyme involved in cellular stress responses, were responsible for her condition.
No therapeutic intervention could be found for the young girl, but the clinicians were able to change treatment protocols for 18 of the affected individuals in the study. In one patient with a newly-described condition that affects mitochondria (the power factories of the cell), the researchers found that treatment with a drug called carglumic acid could correct a dangerous imbalance of metabolites. In another, with sleep problems and seizures, amino acid and vitamin supplements brought the seizures under control and improved other symptoms caused by mutations in a gene called GOT2 that encodes a different mitochondrial enzyme.
Sixty-eight percent of the people genetically analysed in this study received diagnoses—a diagnostic rate much higher than the 16% documented in previous reports using the same sequencing approach to explain unexplained intellectual disorders. Alfadhel credits the comprehensive patient evaluations for much of the high diagnostic yield. This allowed the team to isolate the sequencing of DNA only for individuals who were likely to benefit from the approach.
However, Alfadhel also points to the close collaboration between the clinical physicians and the computational scientists performing the data analyses. “Data sharing and open communication between scientists and researchers across the globe are the keys to maximizing the diagnostic potential of next-generation sequencing and its clinical benefit for patients and their families,” says Alfadhel, who trained at the British Columbia Children's Hospital in Vancouver, Canada, where most of the patients in the study were treated.
This international experience, he adds, will enable “more collaboration and communication between international experts and my team, which will definitely benefit our patients in Saudi Arabia.”
References
Tarailo-Graovac, M., Shyr, C., Ross, C.J., Horvath, G.A., Salvarinova, R. et al. Exome sequencing and the management of neurometabolic disorders. New England Journal of Medicine (2016).| article