9 January 2019
Researchers designed and compared four vaccines against the potentially deadly Middle East respiratory syndrome (MERS) and found one that activates a strong immune response in mice after a single dose.
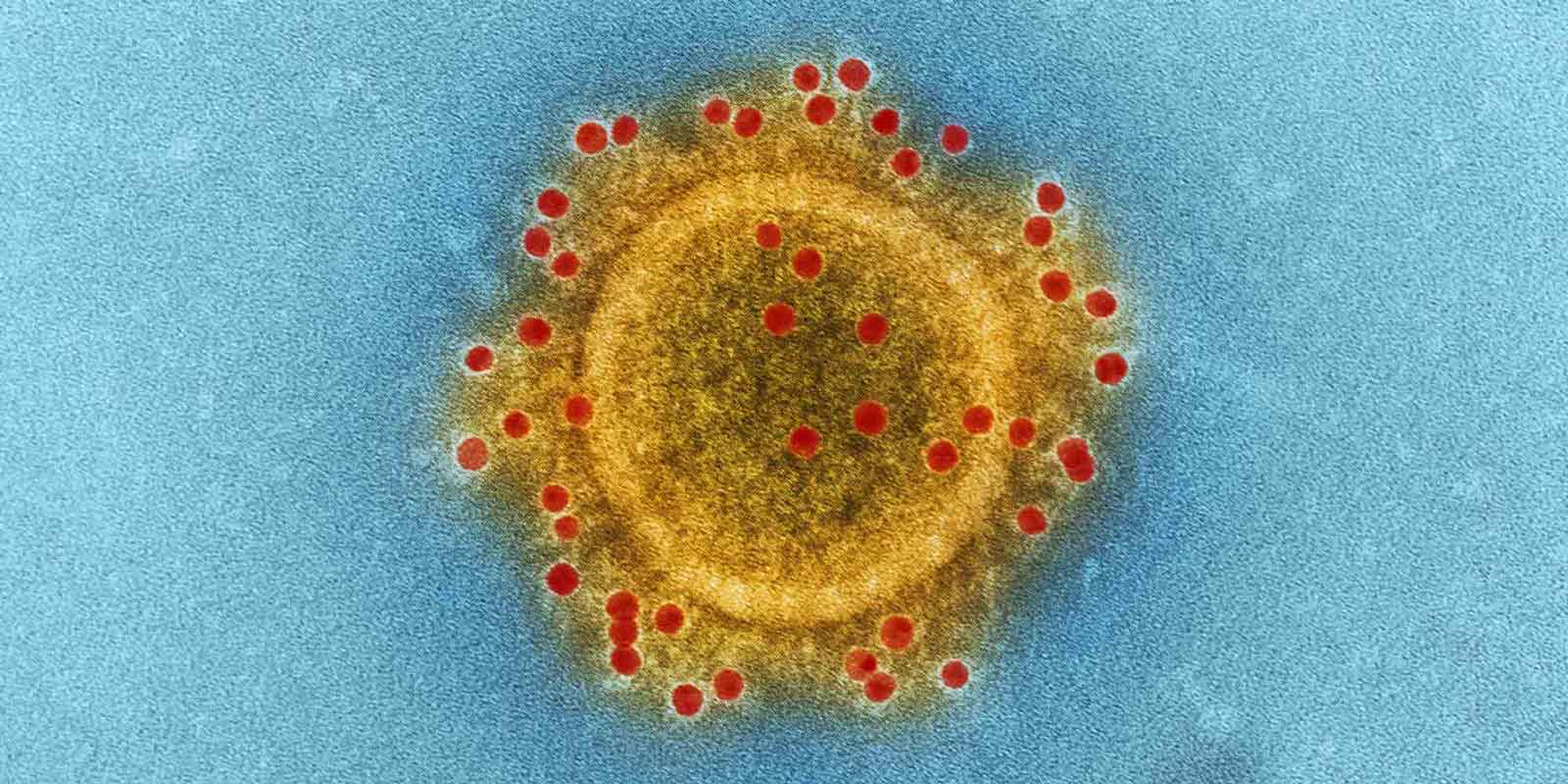
In June 2012, a Saudi patient died from a severe respiratory condition caused by a previously unrecognized coronavirus. Since then, the MERS-CoV virus has been reported in 27 countries and has infected more than 1,900 people, mostly in Saudi Arabia. It is mainly transmitted to humans via dromedary camels and brings a range of clinical outcomes from asymptomatic to severe. Its 40% mortality rate is higher than that caused by severe acute respiratory syndrome (SARS), but no vaccines or therapies have been clinically approved for human or veterinary use.
One approach to tackle MERS is to use vaccines made from unarmed viruses that are efficient in triggering the development of a strong immune response against other dangerous viruses. ‘Viral vector vaccines’ against Ebola, HIV, malaria, tuberculosis, and influenza are already under study.
To date, the only anti-MERS vaccine to be tested in camels is derived from a highly attenuated strain of vaccinia virus, called modified vaccinia Ankara (MVA). It requires two doses to provide partial protection. An ideal vaccine would yield complete, rapid and long-lasting immunity after a single dose.
Researchers from KAIMRC, the UK, and Germany compared MVA with a different viral vector, known as chimpanzee adenovirus Oxford University #1 (ChAdOx1). Previous studies have shown that one dose of ChAdOx1-based vaccine is effective against Rift Valley fever in multiple species, including camels, making it a promising vaccine tool.
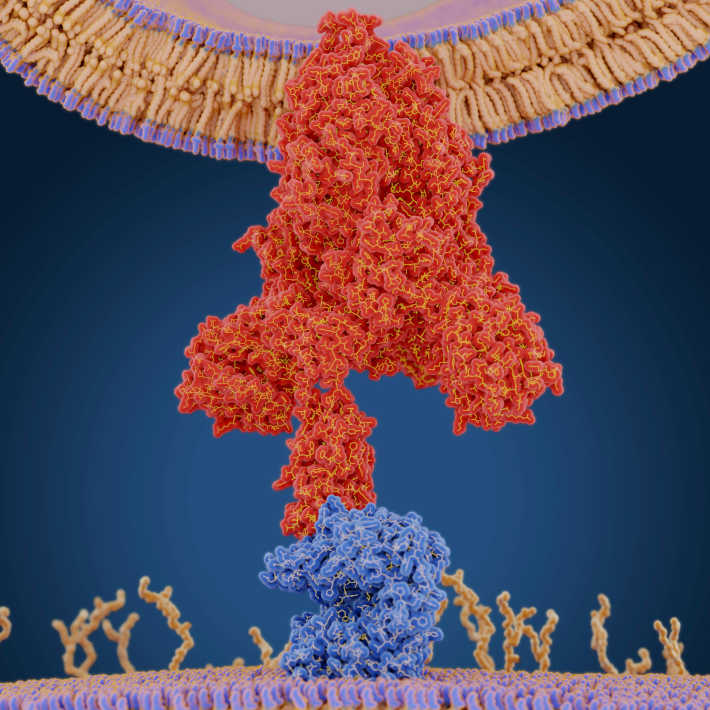
The scientists tested two MVA and two ChAdOx1 MERS vaccines. All of them carried the DNA sequence encoding MERS-CoV’s characteristic spikeshaped surface proteins, which are produced once the viral vector enters host cells. These are then recognized by the host’s immune cells, triggering more defence mechanisms. The production of the spike proteins is dependent on a promoter sequence. The MVA MERS vaccines were designed with one of two promoters, either mH5 or F11, to test which allows the largest production of spike proteins, enhancing the strongest immunity. The two ChAdOx1 MERS vaccines differed for the presence or NATIONAL INSTITUTE OF ALLERGY AND INFECTIOUS DISEASES (NIAID)absence of tissue plasminogen activator (tPA), another strategy to optimize the vaccine’s performance. They found that all these vaccine candidates stimulated the mouse immune system and the type of promoter did not have a significant impact on the results. Strikingly, a single dose of ChAdOx1 MERS with tPA was as immunogenic as two doses of MVA MERS (also containing tPA). The team suggests testing it as a single dose vaccine in dromedary camels, followed by a booster dose of MVA MERS vaccine if needed.
References
- Alharbi, N. K., Padron-Regalado, E., Thompson, C. P., Kupke, A., Wells, D., Sloan, M. A. et al . ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine | article