10 February 2019
Ohio State University researchers in the United States have found that a carrier made from the protein coat of a virus could efficiently deliver genetic material to the spinal cord. This could provide future therapy options for conditions like spinal muscular atrophy.
Spinal muscular atrophy is the leading genetic cause of death for babies and toddlers. The condition causes the breakdown of nerves in the brain and spinal cord that are responsible for supplying muscles. This often leads to muscle weakness, wasting and breathing difficulties. There is no cure for the condition, but delivering genetic material that can promote the survival of motor neurons could be an option.
Lei Cao and co-workers at Ohio State University previously studied a family of viral vectors made from protein coats of the adeno-associated virus in which the DNA sequences were shuffled to make variations of the vector. Adeno-associated virus is naturally occurring and infects humans but is not known to cause disease. It is widely believed to be a safe and powerful vehicle for delivering genetic material into a patient’s body. The researchers found that one of the variations, called Rec2, efficiently delivered genetic material to fatty tissues — a feat that was previously difficult to achieve by adeno-associated virus.
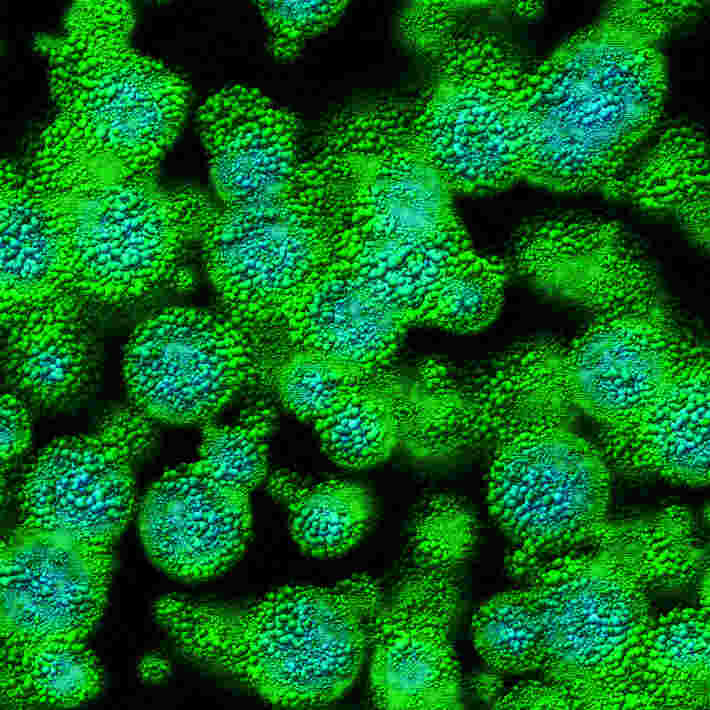
They tested to see if Rec2 or other members in its family were equally efficient at delivering genetic material to the spinal cord. They injected Rec2, Rec3, Rec4 and AAV9 (a common adeno-associated viral vector used in clinical trials for diseases affecting the spinal cord) expressing green fluorescent protein into the spinal cord of adult mice, and then measured the intensity of green fluorescence along the spinal cord three weeks after injection.
Cao and her team found that Rec3 was most efficient in delivering genetic material to the spinal cord. At equal dosage, Rec3 elicited green fluorescence over a longer section of the spinal cord (up to 1.5 cm) than Rec2, Rec4 or AAV9. More importantly, an injection containing as little as 0.4 billion viral particles of Rec3 was enough to generate green fluorescence in 60–90% of cells near the injection site in the spinal cord. For the sake of comparison, AAV9 would require 40 billion viral particles to achieve the same.
The results suggest that Rec3 might be an efficient and cost-effective vehicle vector, because it needs fewer viral particles, for delivering genetic material to the spinal cord. The viral vector may be used, for example, to carry genetic material that induces the secretion of proteins that promote the survival of motor neurons. Through this approach, scientists may one day develop a therapeutic treatment for spinal muscular atrophy.
“One factor that may be prohibitive for patients receiving gene therapy is cost,” says Cao. “If we can design viral vectors that can target specific cell types more efficiently and at lower dosage, life-saving therapeutics will become more accessible.”
References
- Siu, J. J., Queen, N. J., Huang, W., Yin, F. Q., Liu, X. et al. Improved gene delivery to adult mouse spinal cord through the use of engineered hybrid adeno-associated viral serotypes. Gene Therapy 24, 361–369 (2017). | article