11 February 2019
“They can’t eat, they can’t walk, they can’t talk… It’s heart-breaking,” says the University of Edinburgh’s Emma Hall, who over the last seven years has worked with an international cohort to study a hereditary disease that has devastated four families from the UK and Saudi Arabia.
PLAA-associated neurodevelopmental disorder (PLAAND), described for the first time in Hall’s paper, severely disrupts the infant brain and leads to seizures, impaired brain growth, optic nerve degeneration, and a progressive deterioration of muscle control. Affected children die before their sixth birthday.
“It’s an extremely rare disease. We managed to identify just three families in the UK and one in Saudi Arabia. Another study was able to identify two Israeli families with a milder form of the illness,” says Hall. Her group embarked on their project after a colleague identified seven children from three families who shared an unusual clinical presentation without a diagnosis.
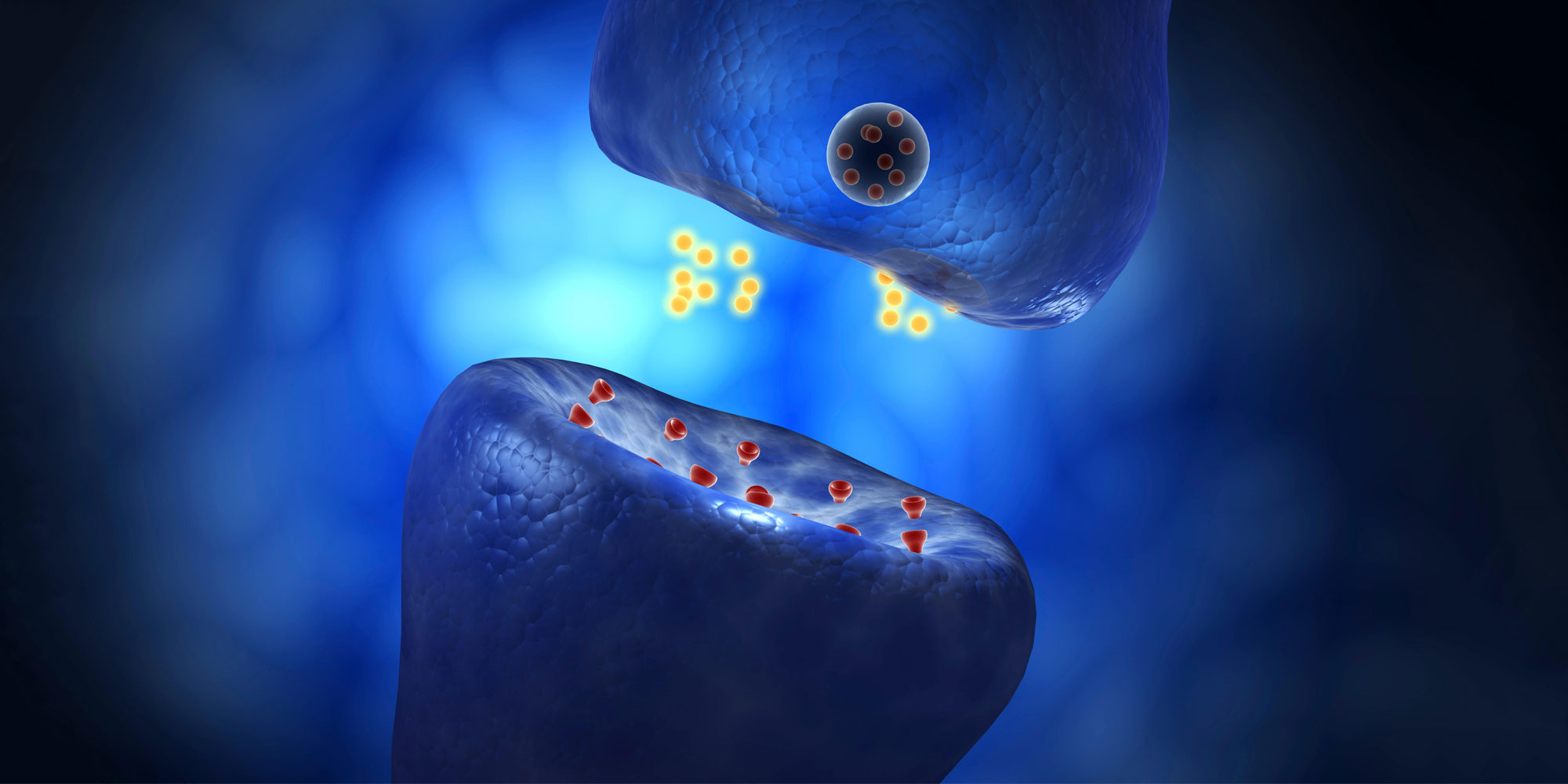
Publishing their findings in The American Journal of Human Genetics, the group sequenced the patients’ DNA and found a shared, near-identical mutation in their genetic code for the protein ‘PLAA.’ Using the pioneering ‘genetic scissor’ technology CRISPR, the researchers designed mice with the exact mutation suffered by the children. Analyses of these mice elucidated PLAA’s critical role in maintaining the brain’s small, specialized gaps, called synapses, across which information flows from one neuron to the next.
“Proteins involved in propagating synaptic signals need to be sorted to the right place so the synapse can re-fire,” explains Pleasantine Mill of the University of Edinburgh. “Similar to baggage tags at the airport, the cell chemically tags proteins to ‘fast-track’ them to their correct destination. We think PLAA acts as the scanner of these tags.”
In children with defective PLAA, trafficking at synapses breaks down, signalling and transport proteins accumulate, and brain cells are rendered unable to develop or communicate correctly. “As a result, some receive too much signal or for too long, others not enough or at the wrong time,” says Mill.
The new insights into PLAA’s function may offer a window into more common conditions, such as Alzheimer’s and Parkinson’s, which also involve protein accumulation at synapses.
“In future, I think we will try to reroute the accumulation of proteins with drugs,” says Hall. “It’s exciting that our studies might be able to shed light on treatments for both this really rare disease, as well as much more common neurological diseases.”
References
- Hall, E. A., Nahorski, M. S., Murray, L. M., Shaheen, R., Perkins, E. et al. PLAA mutations cause a lethal infantile epileptic encephalopathy by disrupting ubiquitin-mediated endolysosomal degradation of synaptic proteins. The American Journal of Human Genetics 100, 706 – 724 (2017). | article