21 April 2019
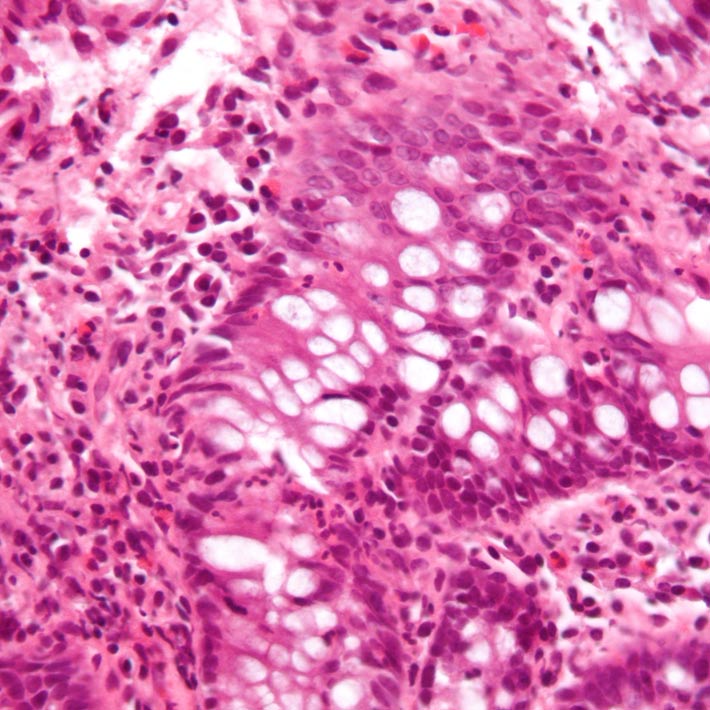
The nucleus needs to be reprogrammed for cells to differentiate into new cell types. During this process, chromatin is seen to be re-organizing itself in a way that is thought to turn on new gene expression programs in the cell. A protein, called beta-actin, is implicated in this process of chromatin remodeling.
Now, a new study shows that beta-actin does play an essential role in regulating which gene programs are turned on in the nucleus during cell differentiation and development. It does this by bringing certain genes closer together at ‘docking stations’ on the inside of the nuclear membrane.
A research team, including Bader Almuzzaini from King Abdullah International Medical Research Center, ‘turned off’ beta-actin in mice embryos to see how this affected fibre- and collagen-producing cells called fibroblasts.
They found that densely packed chromosomal material, called heterochromatin, was not only localized to the inside of the nuclear membrane, as is the case in beta-actin-containing cells, but was also present within the nuclear interior. This indicates that beta-actin plays a role in confining heterochromatin to the nuclear periphery.
Beta-actin is an important component of the so-called ‘chromatin-remodelling complexes’; in particular the one known as Brahma–associated factor (BAF). Cells mobilize these complexes to hamper bonds in chromatin between proteins called histones and DNA. This reconfigures the DNA packaging structure and modifies gene expression. There is evidence that BAF anchors heterochromatin to the nuclear periphery. The scientists suggest that actin is involved in the interaction between heterochromatin and the nuclear membrane, through the regulation of the Brahma-related gene 1 (BRG1) subunit of the BAF complex. Since the team measured a substantial loss of BRG1–chromatin binding in the absence of beta-actin, they suggest that BAF deprived of beta-actin is less efficient in binding and docking heterochromatin, which might explain why it was found in the nuclear interior.
The research sheds some light on the complex and still poorly-understood genetic reprogramming mechanism of cells, and suggests how beta-actin can act at the interplay between nuclear architecture, heterochromatin organization, and regulation of gene expression.
References
Xie, X., Almuzzaini, B., Drou, N., Kremb, S., Yousif, A., et al. β-Actin–dependent global chromatin organization and gene expression programs control cellular identity. The FASEB Journal (2018). | article