17 March 2021
Genetic analysis of two Middle Eastern patients has revealed that mutation of a gene involved in maintaining cellular structure has cascading consequences, leading to impaired immune activity and hyperinflammation.
Mutations that cause cellular processes to go awry can lead to a malfunctioning immune system. When the immune system is working properly, lymphocytes and macrophages target infections so that healthy tissues are not harmed. However, when it malfunctions, the adaptive immune system may overreact, causing severe inflammation, uncontrolled lymphocyte proliferation and weakening of the immune system.
An international team led by Seiamak Bahram from the University of Strasbourg has now reported the discovery of two unrelated patients whose immunity problems were caused by a homozygous mutation. The research team included Saudi researchers Amjad Khan, Wafaa Eyaid, and Fayhan Alroqi from the King Abdullah International Medical Research Center.
The two patients, a 15-month-old baby girl of Iranian origin and an 11-year-old boy from Saudi Arabia, presented symptoms of hyperinflammation, lymphoproliferation and immunodeficiency. Specifically, the girl had fever, mild anaemia, as well as a hugely enlarged spleen, while the boy had fever, recurrent ear infection, sinopulmonary infections, bronchiectasis (widening of airways) and oligemia (reduction of total blood volume).
Both patients were originally diagnosed with hemophagocytic lymphohistiocytosis (HLH), a rare life- threatening congenital condition that is also characterized by symptoms of hyperinflammation, lymphoproliferation and immunodeficiency. However, the researchers noted that the patients also displayed symptoms inconsistent with HLH, such as an absence of cytopenia (reduction of mature blood cells), and the boy did not improve when given an HLH treatment.
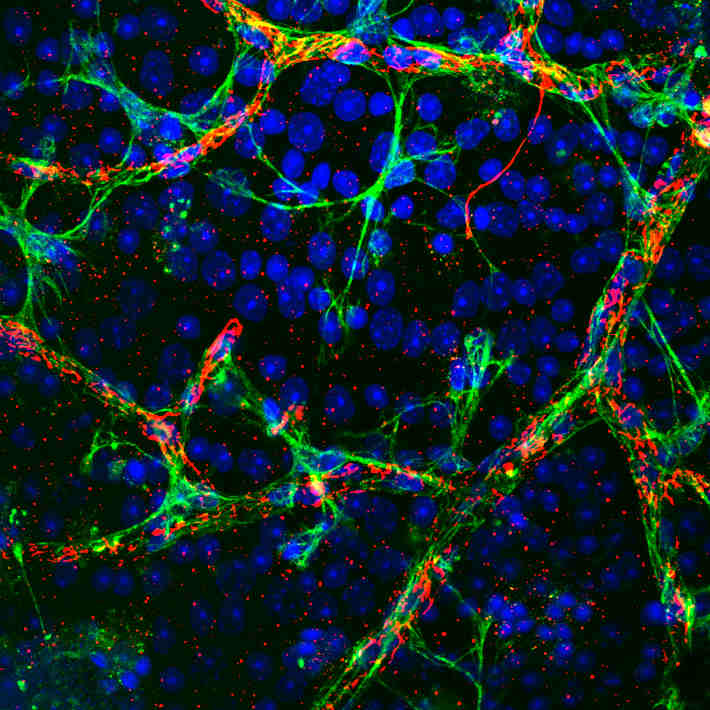
To get to the root of this, the researchers performed whole-exome sequencing and found that both patients, as well as their parents and several siblings in family 2, had a mutation in NCKAP1L, a gene involved in the reorganization of actin cytoskeleton. The cytoskeleton plays a vital role in enabling cells to migrate, contract and keep their shapes. As a result, the patients’ T cells displayed a wide range of abnormalities, including impaired early activation, immune synapse morphology and defective edge formation.
The researchers concluded by classifying the syndrome as a new entity that is similar to yet distinct from HLH and is caused by recessive mutations in NCKAP1L. “NCKAP1L deficiency is a novel disease in humans that leads to immunodeficiency, lymphoproliferation and hyperinflammation with features of hemophagocytic lymphohistiocytosis,” says Alroqi.
References
- Castro, C.N., et al. NCKAP1L defects lead to a novel syndrome combining immunodeficiency, lymphoproliferation, and hyperinflammation. Journal of Experimental Medicine 217, e20192275 (2020).| article